Crystallization and preliminary X-ray analysis of a processive cellobiohydrolase CbCBH48A from Caldicellulosiruptor bescii
Jiao, A., Bai, A., Yan, F., Geng, W.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Glycoside hydrolase family 48 | 656 | Caldicellulosiruptor bescii DSM 6725 | Mutation(s): 0 Gene Names: Athe_1867 EC: 3.2.1.4 | 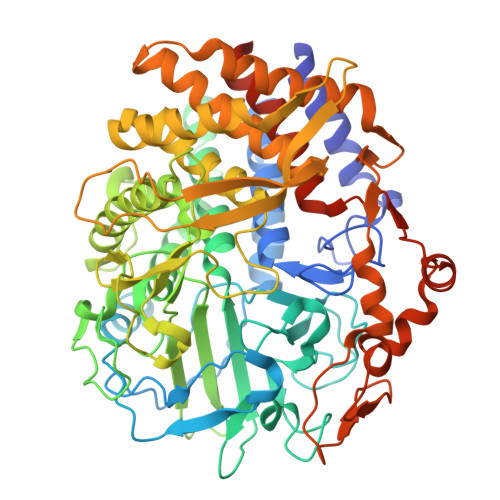 | |
UniProt | |||||
Find proteins for B9MKU7 (Caldicellulosiruptor bescii (strain ATCC BAA-1888 / DSM 6725 / KCTC 15123 / Z-1320)) Explore B9MKU7 Go to UniProtKB: B9MKU7 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | B9MKU7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| BTB Query on BTB | I [auth A] | 2-[BIS-(2-HYDROXY-ETHYL)-AMINO]-2-HYDROXYMETHYL-PROPANE-1,3-DIOL C8 H19 N O5 OWMVSZAMULFTJU-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | C [auth A] D [auth A] E [auth A] F [auth A] G [auth A] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| ID | Chains | Name | Type/Class | 2D Diagram | 3D Interactions |
| PRD_900005 Query on PRD_900005 | B | beta-cellobiose | Oligosaccharide / Metabolism |  | |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 116.313 | α = 90 |
| b = 57.225 | β = 103.38 |
| c = 105.897 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |