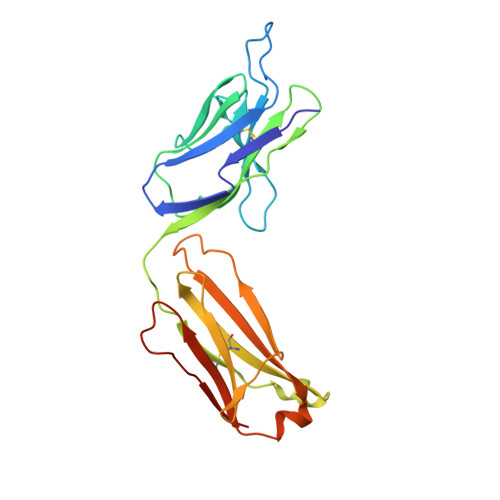
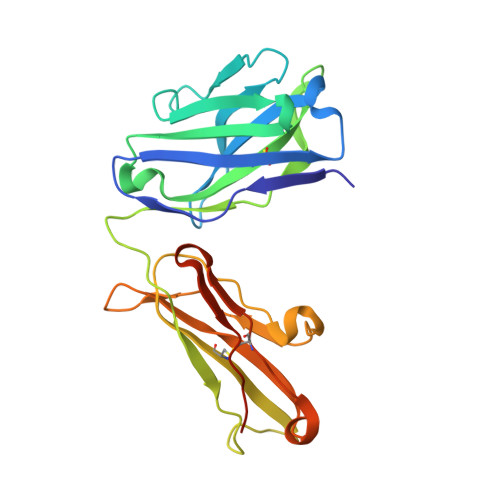
Structural evidence for a constrained conformation of short CDR-L3 in antibodies.
Teplyakov, A., Obmolova, G., Malia, T.J., Luo, J., Gilliland, G.L.(2014) Proteins 82: 1679-1683
- PubMed: 24470236
- DOI: https://doi.org/10.1002/prot.24522
- Primary Citation of Related Structures:
4KQ3, 4KUZ - PubMed Abstract:
Three Fab structures used as targets in the Antibody Modeling Assessment presented a challenge for modeling CDR-L3 due to deviations from the most typical canonical structure. In all three antibodies CDR-L3 has eight residues, which is one residue shorter than usual, and has a conformation that is rarely observed in crystal structures. We analyzed the sequence and structural determinants of this conformation and found that the "short" CDR-L3 is remarkably rigid and retains the conformation in the interactions with antigens and neighboring CDRs.
- Janssen Research & Development, LLC, Spring House, Pennsylvania, 19477.
Organizational Affiliation: