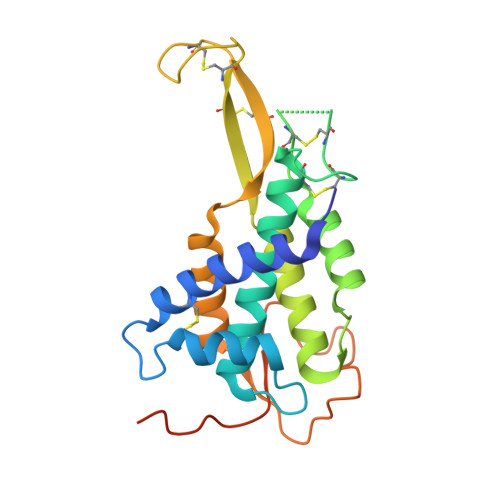
Structural Studies of Wnts and Identification of an LRP6 Binding Site.
Chu, M.L., Ahn, V.E., Choi, H.J., Daniels, D.L., Nusse, R., Weis, W.I.(2013) Structure 21: 1235-1242
- PubMed: 23791946
- DOI: https://doi.org/10.1016/j.str.2013.05.006
- Primary Citation of Related Structures:
4KRR - PubMed Abstract:
Wnts are secreted growth factors that have critical roles in cell fate determination and stem cell renewal. The Wnt/β-catenin pathway is initiated by binding of a Wnt protein to a Frizzled (Fzd) receptor and a coreceptor, LDL receptor-related protein 5 or 6 (LRP5/6). We report the 2.1 Å resolution crystal structure of a Drosophila WntD fragment encompassing the N-terminal domain and the linker that connects it to the C-terminal domain. Differences in the structures of WntD and Xenopus Wnt8, including the positions of a receptor-binding β hairpin and a large solvent-filled cavity in the helical core, indicate conformational plasticity in the N-terminal domain that may be important for Wnt-Frizzled specificity. Structure-based mutational analysis of mouse Wnt3a shows that the linker between the N- and C-terminal domains is required for LRP6 binding. These findings provide important insights into Wnt function and evolution.
- Departments of Structural Biology and Molecular and Cellular Physiology, Stanford University School of Medicine, Stanford, CA 94305, USA.
Organizational Affiliation: