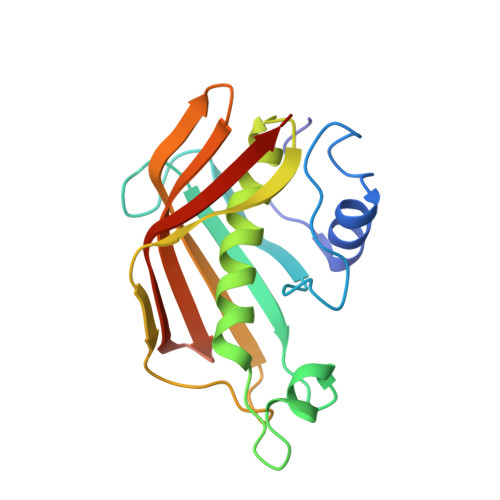
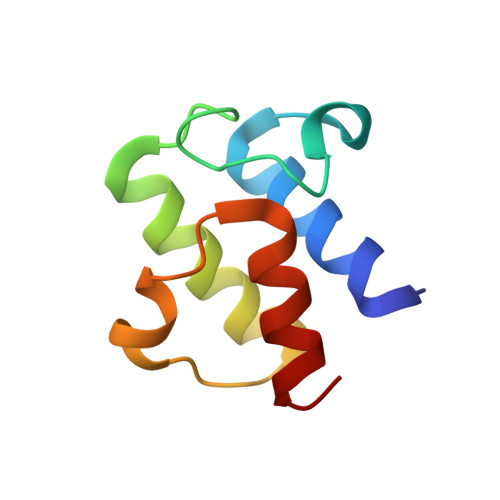
Trapping the dynamic acyl carrier protein in fatty acid biosynthesis.
Nguyen, C., Haushalter, R.W., Lee, D.J., Markwick, P.R., Bruegger, J., Caldara-Festin, G., Finzel, K., Jackson, D.R., Ishikawa, F., O'Dowd, B., McCammon, J.A., Opella, S.J., Tsai, S.C., Burkart, M.D.(2014) Nature 505: 427-431
- PubMed: 24362570
- DOI: https://doi.org/10.1038/nature12810
- Primary Citation of Related Structures:
4KEH - PubMed Abstract:
Acyl carrier protein (ACP) transports the growing fatty acid chain between enzymatic domains of fatty acid synthase (FAS) during biosynthesis. Because FAS enzymes operate on ACP-bound acyl groups, ACP must stabilize and transport the growing lipid chain. ACPs have a central role in transporting starting materials and intermediates throughout the fatty acid biosynthetic pathway. The transient nature of ACP-enzyme interactions impose major obstacles to obtaining high-resolution structural information about fatty acid biosynthesis, and a new strategy is required to study protein-protein interactions effectively. Here we describe the application of a mechanism-based probe that allows active site-selective covalent crosslinking of AcpP to FabA, the Escherichia coli ACP and fatty acid 3-hydroxyacyl-ACP dehydratase, respectively. We report the 1.9 Å crystal structure of the crosslinked AcpP-FabA complex as a homodimer in which AcpP exhibits two different conformations, representing probable snapshots of ACP in action: the 4'-phosphopantetheine group of AcpP first binds an arginine-rich groove of FabA, then an AcpP helical conformational change locks AcpP and FabA in place. Residues at the interface of AcpP and FabA are identified and validated by solution nuclear magnetic resonance techniques, including chemical shift perturbations and residual dipolar coupling measurements. These not only support our interpretation of the crystal structures but also provide an animated view of ACP in action during fatty acid dehydration. These techniques, in combination with molecular dynamics simulations, show for the first time that FabA extrudes the sequestered acyl chain from the ACP binding pocket before dehydration by repositioning helix III. Extensive sequence conservation among carrier proteins suggests that the mechanistic insights gleaned from our studies may be broadly applicable to fatty acid, polyketide and non-ribosomal biosynthesis. Here the foundation is laid for defining the dynamic action of carrier-protein activity in primary and secondary metabolism, providing insight into pathways that can have major roles in the treatment of cancer, obesity and infectious disease.
- 1] Departments of Molecular Biology and Biochemistry, Chemistry, and Pharmaceutical Sciences, University of California, Irvine, California 92697, USA [2].
Organizational Affiliation: