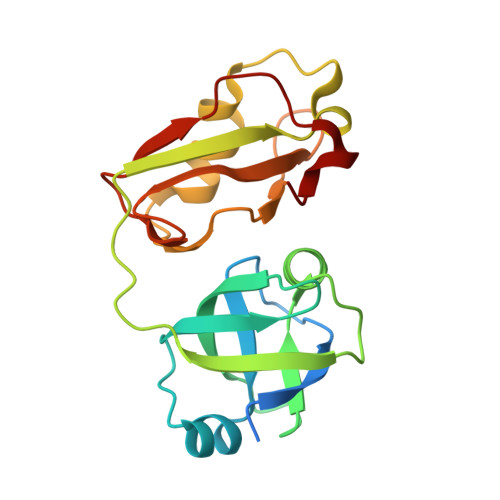
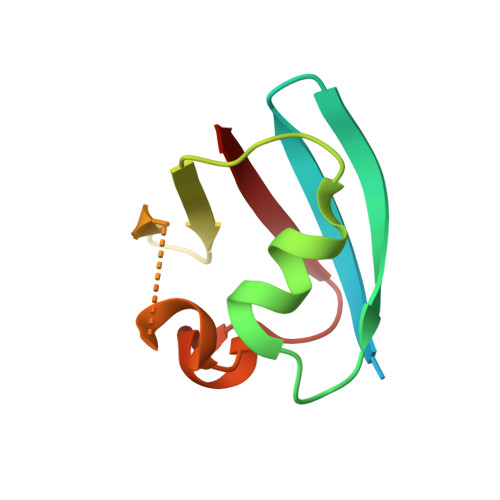
Structural Basis for Ovarian Tumor Domain-containing Protein 1 (OTU1) Binding to p97/Valosin-containing Protein (VCP).
Kim, S.J., Cho, J., Song, E.J., Kim, S.J., Kim, H.M., Lee, K.E., Suh, S.W., Kim, E.E.(2014) J Biological Chem 289: 12264-12274
- PubMed: 24610782
- DOI: https://doi.org/10.1074/jbc.M113.523936
- Primary Citation of Related Structures:
4KDI, 4KDL - PubMed Abstract:
Valosin-containing protein (VCP), also known as p97, is an AAA(+) ATPase that plays an essential role in a broad array of cellular processes including the endoplasmic reticulum-associated degradation (ERAD) pathway. Recently, ERAD-specific deubiquitinating enzymes have been reported to be physically associated with VCP, although the exact mechanism is not yet clear. Among these enzymes is ovarian tumor domain-containing protein 1 (OTU1). Here, we report the structural basis for interaction between VCP and OTU1. The crystal structure of the ubiquitin regulatory X-like (UBXL) domain of OTU1 (UBXLOTU1) complexed to the N-terminal domain of VCP (NVCP) at 1.8-Å resolution reveals that UBXLOTU1 adopts a ubiquitin-like fold and binds at the interface of two subdomains of NVCP using the (39)GYPP(42) loop of UBXLOTU1 with the two prolines in cis- and trans-configurations, respectively. A mutagenesis study shows that this loop is not only critical for the interaction with VCP but also for its role in the ERAD pathway. Negative staining EM shows that one molecule of OTU1 binds to one VCP hexamer, and isothermal titration calorimetry suggests that the two proteins bind with a KD of 0.71 μM. Analytical size exclusion chromatography and isothermal titration calorimetry demonstrates that OTU1 can bind VCP in both the presence and absence of a heterodimer formed by ubiquitin fusion degradation protein 1 and nuclear localization protein 4.
- From the Biomedical Research Institute, Korea Institute of Science and Technology, Seoul 136-791, Republic of Korea.
Organizational Affiliation: