Generation and characterization of a unique reagent that recognizes a panel of recombinant human monoclonal antibody therapeutics in the presence of endogenous human IgG.
Wang, X., Quarmby, V., Ng, C., Chuntharapai, A., Shek, T., Eigenbrot, C., Kelley, R.F., Shia, S., McCutcheon, K., Lowe, J., Leddy, C., Coachman, K., Cain, G., Chu, F., Hotzel, I., Maia, M., Wakshull, E., Yang, J.(2013) MAbs 5: 540-554
- PubMed: 23774668
- DOI: https://doi.org/10.4161/mabs.24822
- Primary Citation of Related Structures:
4K7P - PubMed Abstract:
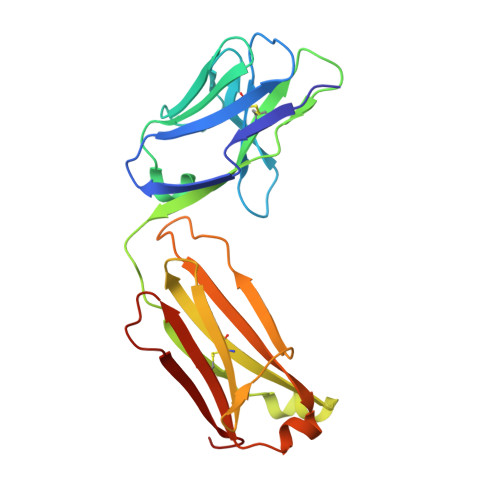
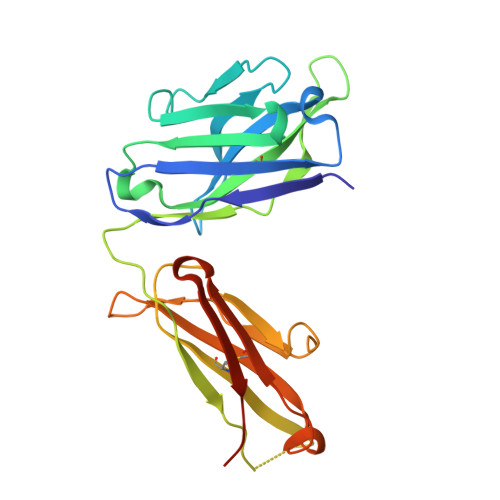
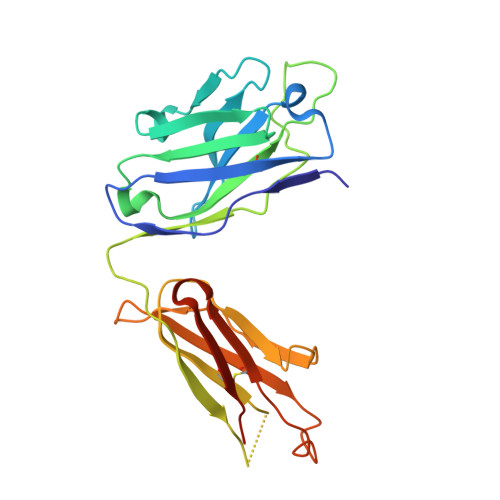
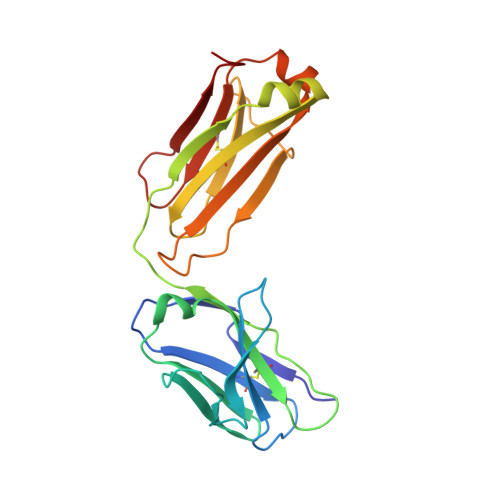
Pharmacokinetic (PK) and immunohistochemistry (IHC) assays are essential to the evaluation of the safety and efficacy of therapeutic monoclonal antibodies (mAb) during drug development. These methods require reagents with a high degree of specificity because low concentrations of therapeutic antibody need to be detected in samples containing high concentrations of endogenous human immunoglobulins. Current assay reagent generation practices are labor-intensive and time-consuming. Moreover, these practices are molecule-specific and so only support one assay for one program at a time. Here, we describe a strategy to generate a unique assay reagent, 10C4, that preferentially recognizes a panel of recombinant human mAbs over endogenous human immunoglobulins. This "panel-specific" feature enables the reagent to be used in PK and IHC assays for multiple structurally-related therapeutic mAbs. Characterization revealed that the 10C4 epitope is conformational, extensive and mainly composed of non-CDR residues. Most key contact residues were conserved among structurally-related therapeutic mAbs, but the combination of these residues exists at low prevalence in endogenous human immunoglobulins. Interestingly, an indirect contact residue on the heavy chain of the therapeutic appears to play a critical role in determining whether or not it can bind to 10C4, but has no affect on target binding. This may allow us to improve the binding of therapeutic mAbs to 10C4 for assay development in the future. Here, for the first time, we present a strategy to develop a panel-specific reagent that can expedite the development of multiple clinical assays for structurally-related therapeutic mAbs.
- BioAnalytical Sciences, Genentech, South San Francisco, CA USA. wang.xiangdan@gene.com
Organizational Affiliation: