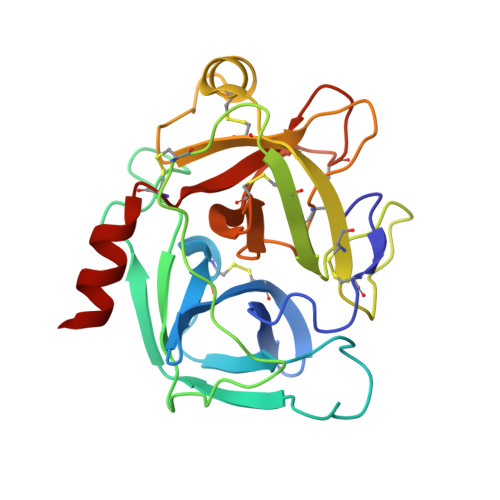
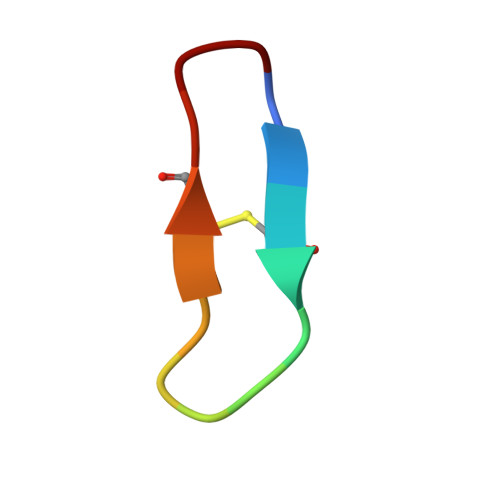
Direct and indirect mechanisms of KLK4 inhibition revealed by structure and dynamics
Riley, B.T., Ilyichova, O., Costa, M.G.S., Porebski, B.T., de Veer, S.J., Swedberg, J.E., Kass, I., Harris, J.M., Hoke, D.E., Buckle, A.M.(2016) Sci Rep 6: 35385-35385
- PubMed: 27767076
- DOI: https://doi.org/10.1038/srep35385
- Primary Citation of Related Structures:
4K1E, 4K8Y, 4KGA - PubMed Abstract:
The kallikrein-related peptidase (KLK) family of proteases is involved in many aspects of human health and disease. One member of this family, KLK4, has been implicated in cancer development and metastasis. Understanding mechanisms of inactivation are critical to developing selective KLK4 inhibitors. We have determined the X-ray crystal structures of KLK4 in complex with both sunflower trypsin inhibitor-1 (SFTI-1) and a rationally designed SFTI-1 derivative to atomic (~1 Å) resolution, as well as with bound nickel. These structures offer a structural rationalization for the potency and selectivity of these inhibitors, and together with MD simulation and computational analysis, reveal a dynamic pathway between the metal binding exosite and the active site, providing key details of a previously proposed allosteric mode of inhibition. Collectively, this work provides insight into both direct and indirect mechanisms of inhibition for KLK4 that have broad implications for the enzymology of the serine protease superfamily, and may potentially be exploited for the design of therapeutic inhibitors.
- Department of Biochemistry and Molecular Biology, Biomedicine Discovery Institute, Monash University, Clayton, Victoria 3800, Australia.
Organizational Affiliation: