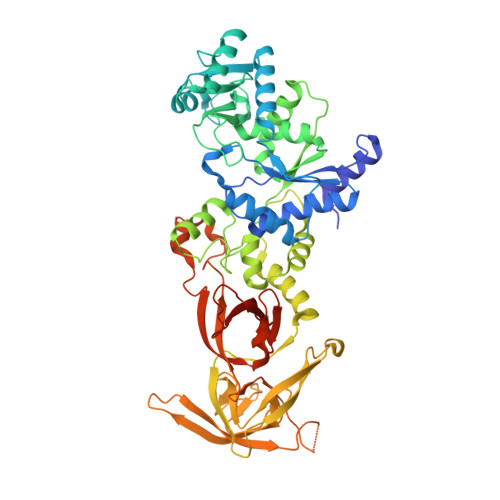
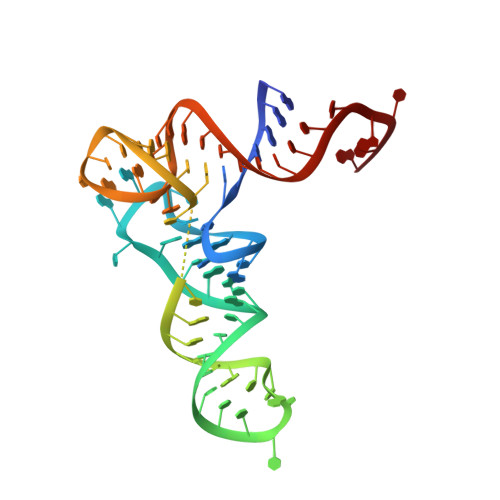
Structural and Mechanistic Basis for Enhanced Translational Efficiency by 2-Thiouridine at the tRNA Anticodon Wobble Position.
Rodriguez-Hernandez, A., Spears, J.L., Gaston, K.W., Limbach, P.A., Gamper, H., Hou, Y.M., Kaiser, R., Agris, P.F., Perona, J.J.(2013) J Mol Biology 425: 3888-3906
- PubMed: 23727144
- DOI: https://doi.org/10.1016/j.jmb.2013.05.018
- Primary Citation of Related Structures:
4JXX, 4JXZ, 4JYZ - PubMed Abstract:
The 2-thiouridine (s(2)U) at the wobble position of certain bacterial and eukaryotic tRNAs enhances aminoacylation kinetics, assists proper codon-anticodon base pairing at the ribosome A-site, and prevents frameshifting during translation. By mass spectrometry of affinity-purified native Escherichia coli tRNA1(Gln)UUG, we show that the complete modification at the wobble position 34 is 5-carboxyaminomethyl-2-thiouridine (cmnm(5)s(2)U). The crystal structure of E. coli glutaminyl-tRNA synthetase (GlnRS) bound to native tRNA1(Gln) and ATP demonstrates that cmnm(5)s(2)U34 improves the order of a previously unobserved 11-amino-acid surface loop in the distal β-barrel domain of the enzyme and imparts other local rearrangements of nearby amino acids that create a binding pocket for the 2-thio moiety. Together with previously solved structures, these observations explain the degenerate recognition of C34 and modified U34 by GlnRS. Comparative pre-steady-state aminoacylation kinetics of native tRNA1(Gln), synthetic tRNA1(Gln) containing s(2)U34 as sole modification, and unmodified wild-type and mutant tRNA1(Gln) and tRNA2(Gln) transcripts demonstrates that the exocyclic sulfur moiety improves tRNA binding affinity to GlnRS 10-fold compared with the unmodified transcript and that an additional fourfold improvement arises from the presence of the cmnm(5) moiety. Measurements of Gln-tRNA(Gln) interactions at the ribosome A-site show that the s(2)U modification enhances binding affinity to the glutamine codons CAA and CAG and increases the rate of GTP hydrolysis by E. coli EF-Tu by fivefold.
- Department of Chemistry, Portland State University, PO Box 751, Portland, OR 97207, USA; Department of Biochemistry and Molecular Biology, Oregon Health and Sciences University, 3181 Southwest Sam Jackson Park Road, Portland, OR 97239, USA.
Organizational Affiliation: