Importance of the peptide scaffold of drugs that target the hepatitis C virus NS3 protease and its crucial bioactive conformation and dynamic factors.
LaPlante, S., Lemke, C.T.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Polyprotein | 186 | Hepatitis C virus genotype 2 | Mutation(s): 0 Gene Names: POLG_HCVJA EC: 3.4.21.98 | 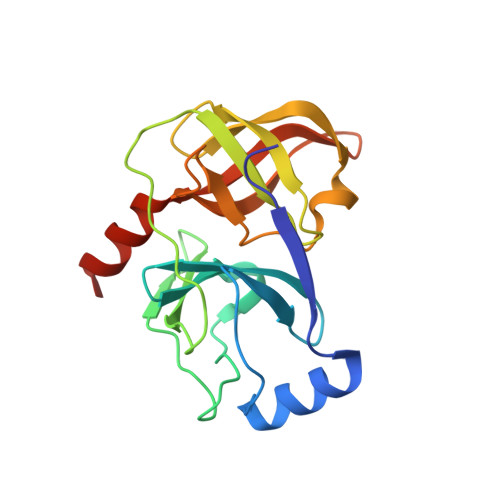 | |
UniProt | |||||
Find proteins for Q81817 (Hepatitis C virus genotype 2) Explore Q81817 Go to UniProtKB: Q81817 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q81817 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| HCV non-structural protein 4A | 17 | N/A | Mutation(s): 0 | 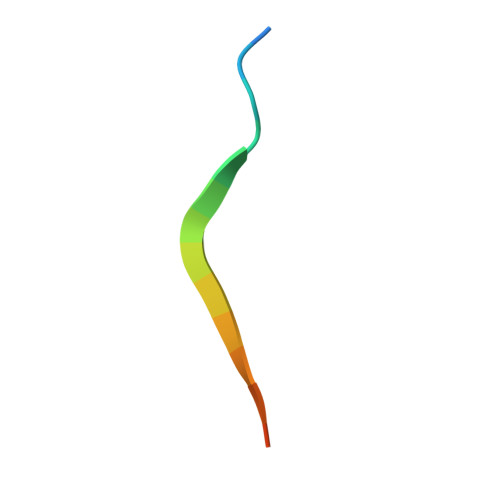 | |
UniProt | |||||
Find proteins for P26662 (Hepatitis C virus genotype 1b (isolate Japanese)) Explore P26662 Go to UniProtKB: P26662 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P26662 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ZN Query on ZN | G [auth A], I [auth B] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| NA Query on NA | H [auth A], J [auth B] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 94.123 | α = 90 |
| b = 94.123 | β = 90 |
| c = 83.236 | γ = 120 |
| Software Name | Purpose |
|---|---|
| MAR345dtb | data collection |
| PHENIX | model building |
| PHENIX | refinement |
| d*TREK | data reduction |
| d*TREK | data scaling |
| PHENIX | phasing |