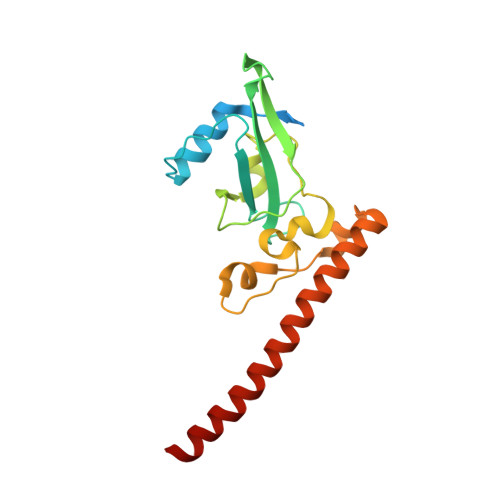
A Unique Spumavirus Gag N-terminal Domain with Functional Properties of Orthoretroviral Matrix and Capsid.
Goldstone, D.C., Flower, T.G., Ball, N.J., Sanz-Ramos, M., Yap, M.W., Ogrodowicz, R.W., Stanke, N., Reh, J., Lindemann, D., Stoye, J.P., Taylor, I.A.(2013) PLoS Pathog 9: e1003376-e1003376
- PubMed: 23675305
- DOI: https://doi.org/10.1371/journal.ppat.1003376
- Primary Citation of Related Structures:
4JMR, 4JNH - PubMed Abstract:
The Spumaretrovirinae, or foamyviruses (FVs) are complex retroviruses that infect many species of monkey and ape. Although FV infection is apparently benign, trans-species zoonosis is commonplace and has resulted in the isolation of the Prototypic Foamy Virus (PFV) from human sources and the potential for germ-line transmission. Despite little sequence homology, FV and orthoretroviral Gag proteins perform equivalent functions, including genome packaging, virion assembly, trafficking and membrane targeting. In addition, PFV Gag interacts with the FV Envelope (Env) protein to facilitate budding of infectious particles. Presently, there is a paucity of structural information with regards FVs and it is unclear how disparate FV and orthoretroviral Gag molecules share the same function. Therefore, in order to probe the functional overlap of FV and orthoretroviral Gag and learn more about FV egress and replication we have undertaken a structural, biophysical and virological study of PFV-Gag. We present the crystal structure of a dimeric amino terminal domain from PFV, Gag-NtD, both free and in complex with the leader peptide of PFV Env. The structure comprises a head domain together with a coiled coil that forms the dimer interface and despite the shared function it is entirely unrelated to either the capsid or matrix of Gag from other retroviruses. Furthermore, we present structural, biochemical and virological data that reveal the molecular details of the essential Gag-Env interaction and in addition we also examine the specificity of Trim5α restriction of PFV. These data provide the first information with regards to FV structural proteins and suggest a model for convergent evolution of gag genes where structurally unrelated molecules have become functionally equivalent.
- Division of Molecular Structure, MRC National Institute for Medical Research, the Ridgeway, Mill Hill, London, United Kingdom.
Organizational Affiliation: