Discovery of Novel Small-Molecule HIV-1 Replication Inhibitors That Stabilize Capsid Complexes.
Lamorte, L., Titolo, S., Lemke, C.T., Goudreau, N., Mercier, J.F., Wardrop, E., Shah, V.B., von Schwedler, U.K., Langelier, C., Banik, S.S., Aiken, C., Sundquist, W.I., Mason, S.W.(2013) Antimicrob Agents Chemother 57: 4622-4631
- PubMed: 23817385
- DOI: https://doi.org/10.1128/AAC.00985-13
- Primary Citation of Related Structures:
4J93 - PubMed Abstract:
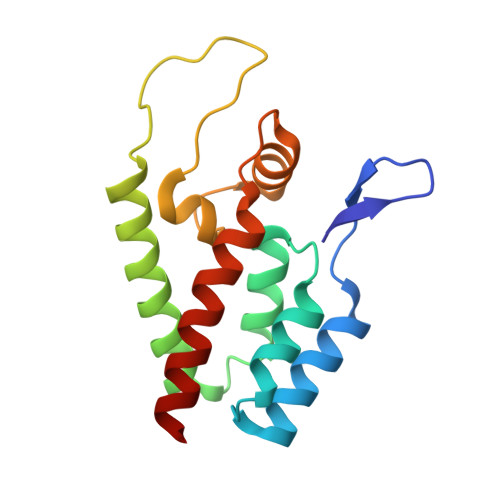
The identification of novel antiretroviral agents is required to provide alternative treatment options for HIV-1-infected patients. The screening of a phenotypic cell-based viral replication assay led to the identification of a novel class of 4,5-dihydro-1H-pyrrolo[3,4-c]pyrazol-6-one (pyrrolopyrazolone) HIV-1 inhibitors, exemplified by two compounds: BI-1 and BI-2. These compounds inhibited early postentry stages of viral replication at a step(s) following reverse transcription but prior to 2 long terminal repeat (2-LTR) circle formation, suggesting that they may block nuclear targeting of the preintegration complex. Selection of viruses resistant to BI-2 revealed that substitutions at residues A105 and T107 within the capsid (CA) amino-terminal domain (CANTD) conferred high-level resistance to both compounds, implicating CA as the antiviral target. Direct binding of BI-1 and/or BI-2 to CANTD was demonstrated using isothermal titration calorimetry and nuclear magnetic resonance (NMR) chemical shift titration analyses. A high-resolution crystal structure of the BI-1:CANTD complex revealed that the inhibitor bound within a recently identified inhibitor binding pocket (CANTD site 2) between CA helices 4, 5, and 7, on the surface of the CANTD, that also corresponds to the binding site for the host factor CPSF-6. The functional consequences of BI-1 and BI-2 binding differ from previously characterized inhibitors that bind the same site since the BI compounds did not inhibit reverse transcription but stabilized preassembled CA complexes. Hence, this new class of antiviral compounds binds CA and may inhibit viral replication by stabilizing the viral capsid.
- Department of Biological Sciences.
Organizational Affiliation: