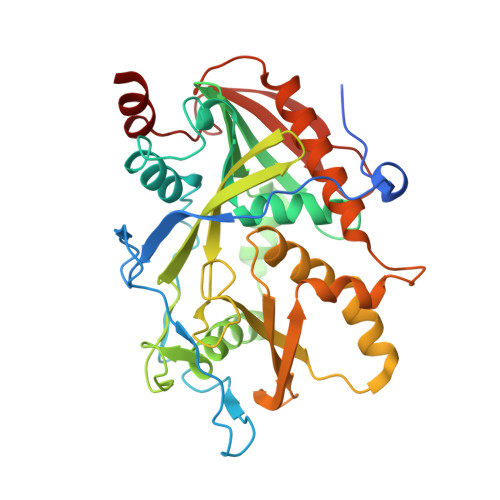
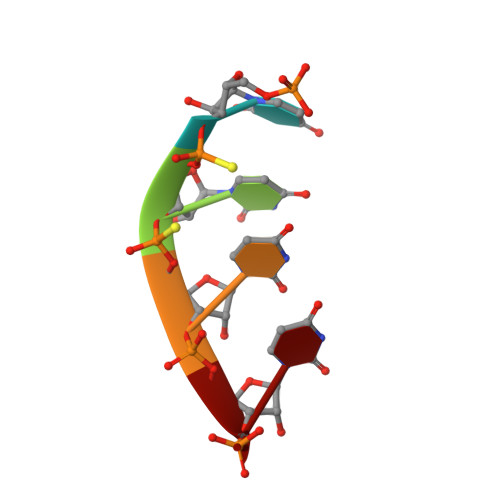
A mammalian pre-mRNA 5' end capping quality control mechanism and an unexpected link of capping to pre-mRNA processing.
Jiao, X., Chang, J.H., Kilic, T., Tong, L., Kiledjian, M.(2013) Mol Cell 50: 104-115
- PubMed: 23523372
- DOI: https://doi.org/10.1016/j.molcel.2013.02.017
- Primary Citation of Related Structures:
4J7L, 4J7M, 4J7N - PubMed Abstract:
Recently, we reported that two homologous yeast proteins, Rai1 and Dxo1, function in a quality control mechanism to clear cells of incompletely 5' end-capped messenger RNAs (mRNAs). Here, we report that their mammalian homolog, Dom3Z (referred to as DXO), possesses pyrophosphohydrolase, decapping, and 5'-to-3' exoribonuclease activities. Surprisingly, we found that DXO preferentially degrades defectively capped pre-mRNAs in cells. Additional studies show that incompletely capped pre-mRNAs are inefficiently spliced at all introns, a fact that contrasts with current understanding, and are also poorly cleaved for polyadenylation. Crystal structures of DXO in complex with substrate mimic and products at a resolution of up to 1.5Å provide elegant insights into the catalytic mechanism and molecular basis for their three apparently distinct activities. Our data reveal a pre-mRNA 5' end capping quality control mechanism in mammalian cells, indicating DXO as the central player for this mechanism, and demonstrate an unexpected intimate link between proper 5' end capping and subsequent pre-mRNA processing.
- Department Cell Biology and Neuroscience, Rutgers University, Piscataway, NJ 08854, USA.
Organizational Affiliation: