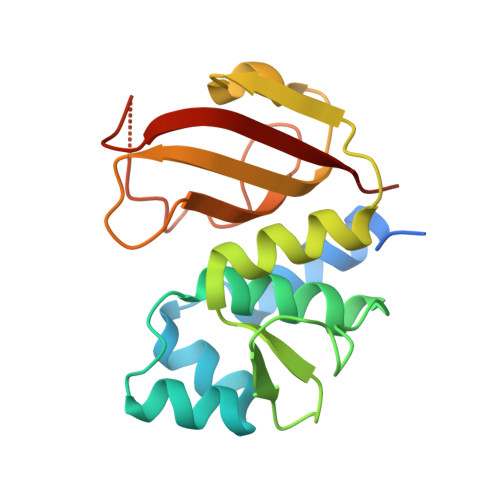
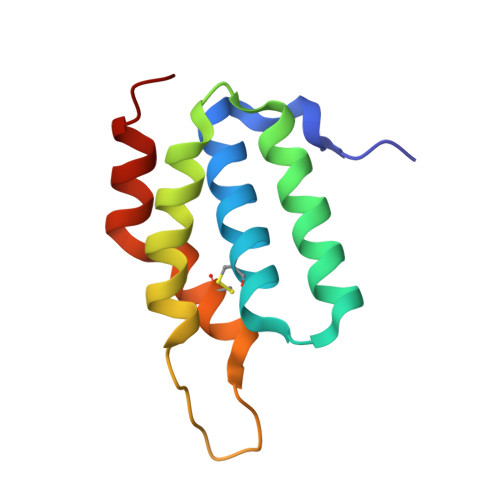
Structural Insights into the Effector - Immunity System Tae4/Tai4 from Salmonella typhimurium.
Benz, J., Reinstein, J., Meinhart, A.(2013) PLoS One 8: e67362-e67362
- PubMed: 23826277
- DOI: https://doi.org/10.1371/journal.pone.0067362
- Primary Citation of Related Structures:
4J30, 4J32 - PubMed Abstract:
Type-6-secretion systems of Gram-negative bacteria are widely distributed needle-like multi-protein complexes that are involved in microbial defense mechanisms. During bacterial competition these injection needles dispense effector proteins into the periplasm of competing bacteria where they induce degradation of the peptidoglycan scaffold and lead to cell lysis. Donor cells co-produce immunity proteins and shuttle them into their own periplasm to prevent accidental toxication by siblings. Recently, a plethora of previously unidentified hydrolases have been suggested to be peptidoglycan degrading amidases. These hydrolases are part of effector/immunity pairs that have been associated with bacterial warfare by type-6-secretion systems. The Tae4 and Tai4 operon encoded by Salmonella typhimurium is one of these newly discovered effector/immunity pairs. The Tae4 effector proteins induce cell lysis by cleaving the γ-D-glutamyl-L-meso-diaminopimelic acid amide bond of acceptor stem muropeptides of the Gram-negative peptidoglycan. Although homologues of the Tae4/Tai4 system have been identified in various different pathogens, little is known about the functional mechanism of effector protein activity and their inhibition by the cognate immunity proteins. Here, we present the high-resolution crystal structure of the effector Tae4 of S. typhimurium in complex with its immunity protein Tai4. We show that Tae4 contains a classical NlpC/P60-peptidase core which is common to other effector proteins of the type-6-secretion system. However, Tae4 has unique structural features that are exclusively conserved within the family of Tae4 effectors and which are important for the substrate specificity. Most importantly, we show that although the overall structure of Tai4 is different to previously described immunity proteins, the essential mode of enzyme inhibition is conserved. Additionally, we provide evidence that inhibition in the Tae4/Tai4 heterotetramer relies on a central Tai4 dimer in order to acquire functionality.
- Department of Biomolecular Mechanisms, Max Planck Institute for Medical Research, Heidelberg, Germany.
Organizational Affiliation: