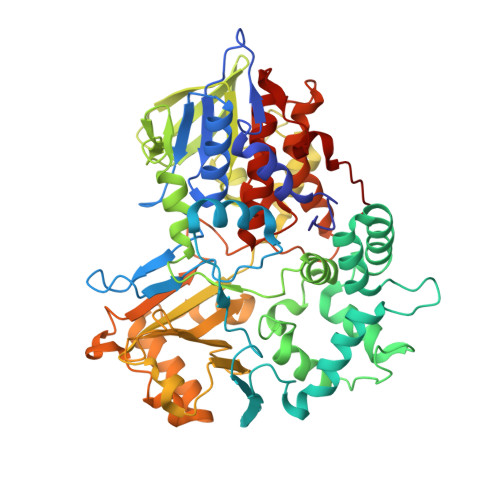
Structure of the flavoprotein tryptophan 2-monooxygenase, a key enzyme in the formation of galls in plants.
Gaweska, H.M., Taylor, A.B., Hart, P.J., Fitzpatrick, P.F.(2013) Biochemistry 52: 2620-2626
- PubMed: 23521653
- DOI: https://doi.org/10.1021/bi4001563
- Primary Citation of Related Structures:
4IV9 - PubMed Abstract:
The flavoprotein tryptophan 2-monooxygenase catalyzes the oxidative decarboxylation of tryptophan to yield indole-3-acetamide. This is the initial step in the biosynthesis of the plant growth hormone indole-acetic acid by bacterial pathogens that cause crown gall and related diseases. The structure of the enzyme from Pseudomonas savastanoi has been determined by X-ray diffraction methods to a resolution of 1.95 Å. The overall structure of the protein shows that it has the same fold as members of the monoamine oxidase family of flavoproteins, with the greatest similarities to the l-amino acid oxidases. The location of bound indole-3-acetamide in the active site allows identification of residues responsible for substrate binding and specificity. Two residues in the enzyme are conserved in all members of the monoamine oxidase family, Lys365 and Trp466. The K365M mutation decreases the kcat and kcat/KTrp values by 60000- and 2 million-fold, respectively. The deuterium kinetic isotope effect increases to 3.2, consistent with carbon-hydrogen bond cleavage becoming rate-limiting in the mutant enzyme. The W466F mutation decreases the kcat value <2-fold and the kcat/KTrp value only 5-fold, while the W466M mutation results in an enzyme lacking flavin and detectable activity. This is consistent with a role for Trp466 in maintaining the structure of the flavin-binding site in the more conserved FAD domain.
- Department of Biochemistry, University of Texas Health Science Center, San Antonio, Texas 78229, USA.
Organizational Affiliation: