In Human Pseudouridine Synthase 1 (hPus1), a C-Terminal Helical Insert Blocks tRNA from Binding in the Same Orientation as in the Pus1 Bacterial Homologue TruA, Consistent with Their Different Target Selectivities.
Czudnochowski, N., Wang, A.L., Finer-Moore, J., Stroud, R.M.(2013) J Mol Biology 425: 3875-3887
- PubMed: 23707380
- DOI: https://doi.org/10.1016/j.jmb.2013.05.014
- Primary Citation of Related Structures:
4IQM, 4ITS, 4J37 - PubMed Abstract:
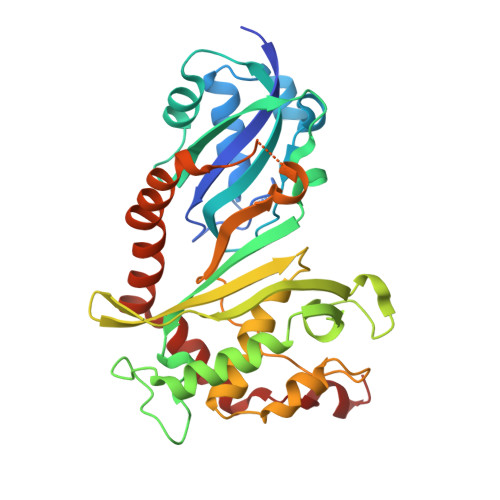
Human pseudouridine (Ψ) synthase Pus1 (hPus1) modifies specific uridine residues in several non-coding RNAs: tRNA, U2 spliceosomal RNA, and steroid receptor activator RNA. We report three structures of the catalytic core domain of hPus1 from two crystal forms, at 1.8Å resolution. The structures are the first of a mammalian Ψ synthase from the set of five Ψ synthase families common to all kingdoms of life. hPus1 adopts a fold similar to bacterial Ψ synthases, with a central antiparallel β-sheet flanked by helices and loops. A flexible hinge at the base of the sheet allows the enzyme to open and close around an electropositive active-site cleft. In one crystal form, a molecule of Mes [2-(N-morpholino)ethane sulfonic acid] mimics the target uridine of an RNA substrate. A positively charged electrostatic surface extends from the active site towards the N-terminus of the catalytic domain, suggesting an extensive binding site specific for target RNAs. Two α-helices C-terminal to the core domain, but unique to hPus1, extend along the back and top of the central β-sheet and form the walls of the RNA binding surface. Docking of tRNA to hPus1 in a productive orientation requires only minor conformational changes to enzyme and tRNA. The docked tRNA is bound by the electropositive surface of the protein employing a completely different binding mode than that seen for the tRNA complex of the Escherichia coli homologue TruA.
- Department of Biochemistry and Biophysics, University of California San Francisco, San Francisco, CA 94158, USA.
Organizational Affiliation: