Structure Analysis of Ubc13 Inactivation
Fu, P., Jin, M., Zhang, X., Xu, L., Xia, Z., Zhu, Y.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ORF169b | 214 | Shigella flexneri | Mutation(s): 1 Gene Names: CP0209, ORF169b, OspI | 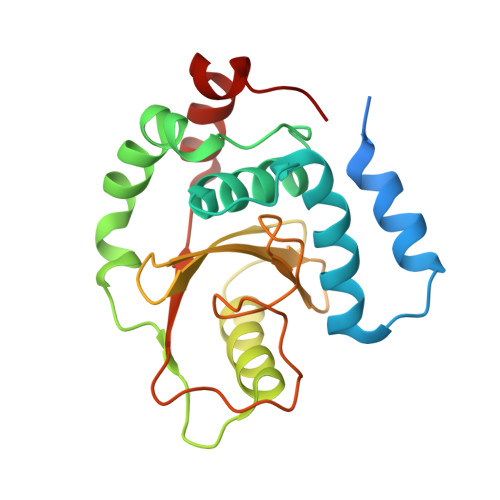 | |
UniProt | |||||
Find proteins for Q8VSD5 (Shigella flexneri) Explore Q8VSD5 Go to UniProtKB: Q8VSD5 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8VSD5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ubiquitin-conjugating enzyme E2 N | 172 | Homo sapiens | Mutation(s): 0 Gene Names: BLU, Ubc13, UBE2N EC: 6.3.2.19 (PDB Primary Data), 2.3.2.23 (UniProt) | 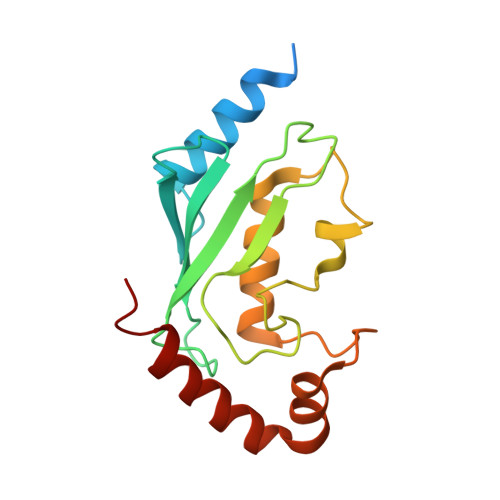 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P61088 (Homo sapiens) Explore P61088 Go to UniProtKB: P61088 | |||||
PHAROS: P61088 GTEx: ENSG00000177889 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P61088 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 119.06 | α = 90 |
| b = 119.06 | β = 90 |
| c = 69.735 | γ = 120 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data collection |
| PHASER | phasing |
| CNS | refinement |
| HKL-2000 | data reduction |
| SCALEPACK | data scaling |