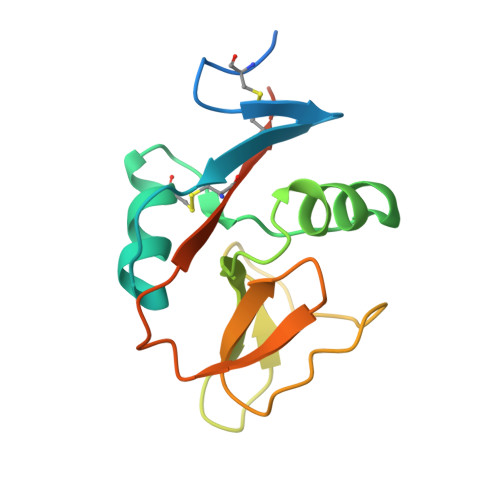
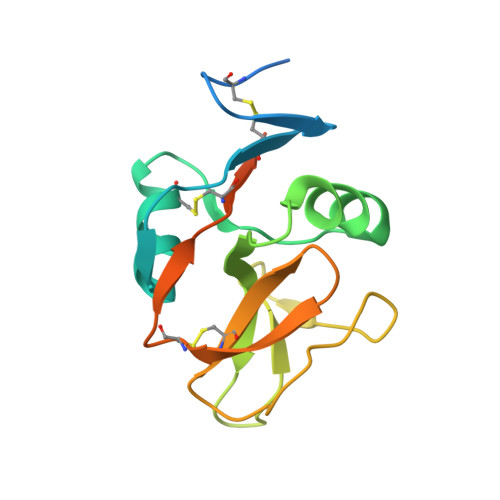
Structure of NKp65 bound to its keratinocyte ligand reveals basis for genetically linked recognition in natural killer gene complex.
Li, Y., Wang, Q., Chen, S., Brown, P.H., Mariuzza, R.A.(2013) Proc Natl Acad Sci U S A 110: 11505-11510
- PubMed: 23803857
- DOI: https://doi.org/10.1073/pnas.1303300110
- Primary Citation of Related Structures:
4IOP - PubMed Abstract:
The natural killer (NK) gene complex (NKC) encodes numerous C-type lectin-like receptors that govern the activity of NK cells. Although some of these receptors (Ly49s, NKG2D, CD94/NKG2A) recognize MHC or MHC-like molecules, others (Nkrp1, NKRP1A, NKp80, NKp65) instead bind C-type lectin-like ligands to which they are genetically linked in the NKC. To understand the basis for this recognition, we determined the structure of human NKp65, an activating receptor implicated in the immunosurveillance of skin, bound to its NKC-encoded ligand keratinocyte-associated C-type lectin (KACL). Whereas KACL forms a homodimer resembling other C-type lectin-like dimers, NKp65 is monomeric. The binding mode in the NKp65-KACL complex, in which a monomeric receptor engages a dimeric ligand, is completely distinct from those used by Ly49s, NKG2D, or CD94/NKG2A. The structure explains the exceptionally high affinity of the NKp65-KACL interaction compared with other cell-cell interaction pairs (KD = 6.7 × 10(-10) M), which may compensate for the monomeric nature of NKp65 to achieve cell activation. This previously unreported structure of an NKC-encoded receptor-ligand complex, coupled with mutational analysis of the interface, establishes a docking template that is directly applicable to other genetically linked pairs in the NKC, including Nkrp1-Clr, NKRP1A-LLT1, and NKp80-AICL.
- W. M. Keck Laboratory for Structural Biology, University of Maryland Institute for Bioscience and Biotechnology Research, Rockville, MD 20850, USA.
Organizational Affiliation: