Spatial Organization of Subunits within the Phenylacetyl-CoA Monooxygenase Complex
Grishin, A.M., Ajamian, E., Tao, L., Bostina, M., Zhang, L., Trempe, J., Menard, R., Rouiller, I., Cygler, M.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 1,2-phenylacetyl-CoA epoxidase, subunit A | 311 | Escherichia coli str. K-12 substr. MG1655 | Mutation(s): 2 Gene Names: b1388, JW1383, paaA, ydbO EC: 1.14.13.149 | 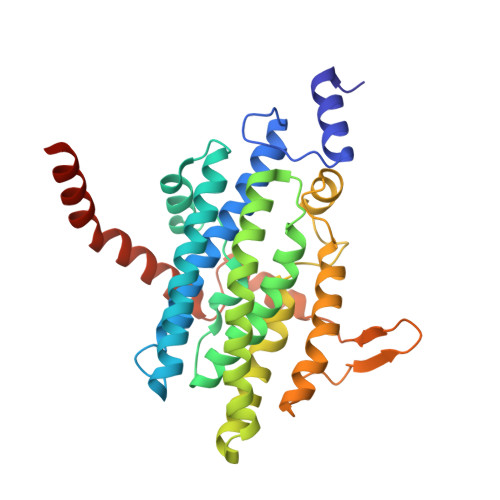 | |
UniProt | |||||
Find proteins for P76077 (Escherichia coli (strain K12)) Explore P76077 Go to UniProtKB: P76077 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P76077 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 1,2-phenylacetyl-CoA epoxidase, subunit C | 259 | Escherichia coli str. K-12 substr. MG1655 | Mutation(s): 0 Gene Names: b1390, JW1385, paaC, ydbP EC: 1.14.13 | 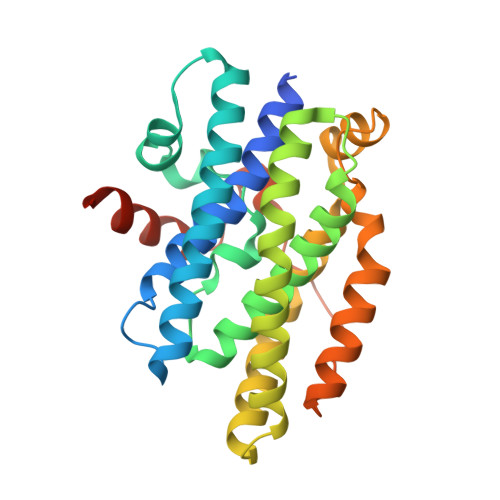 | |
UniProt | |||||
Find proteins for P76079 (Escherichia coli (strain K12)) Explore P76079 Go to UniProtKB: P76079 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P76079 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| BYC Query on BYC | D [auth A] | benzoyl coenzyme A C28 H40 N7 O17 P3 S VEVJTUNLALKRNO-TYHXJLICSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 77.595 | α = 90 |
| b = 77.595 | β = 90 |
| c = 304.692 | γ = 90 |
| Software Name | Purpose |
|---|---|
| SCALEPACK | data scaling |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| MxDC | data collection |
| DENZO | data reduction |
| PHENIX | refinement |