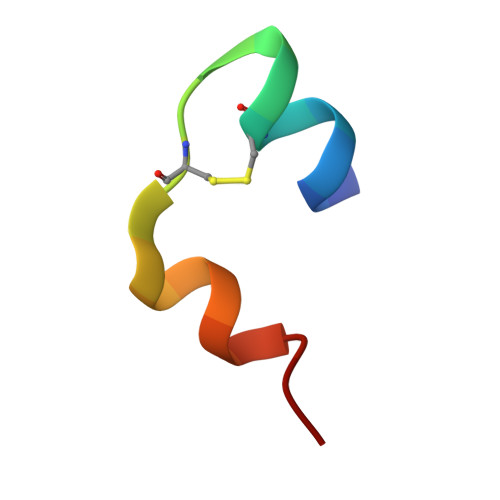
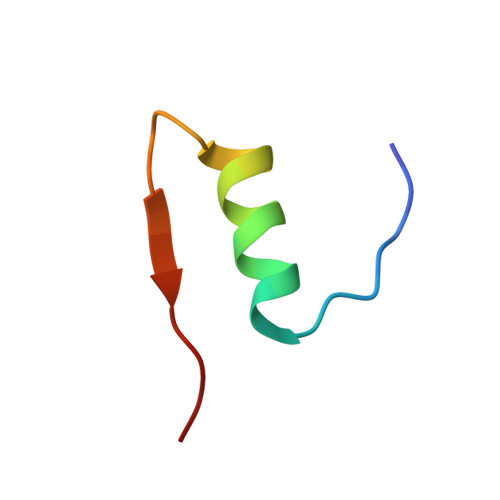
High-resolution powder X-ray data reveal the T6 hexameric form of bovine insulin
Margiolaki, I., Giannopoulou, A.E., Wright, J.P., Knight, L., Norrman, M., Schluckebier, G., Fitch, A.N., Von Dreele, R.B.(2013) Acta Crystallogr D Biol Crystallogr 69: 978-990
- PubMed: 23695242
- DOI: https://doi.org/10.1107/S0907444913003867
- Primary Citation of Related Structures:
4IDW - PubMed Abstract:
A series of bovine insulin samples were obtained as 14 polycrystalline precipitates at room temperature in the pH range 5.0-7.6. High-resolution powder X-ray diffraction data were collected to reveal the T6 hexameric insulin form. Sample homogeneity and reproducibility were verified by additional synchrotron measurements using an area detector. Pawley analyses of the powder patterns displayed pH- and radiation-induced anisotropic lattice modifications. The pronounced anisotropic lattice variations observed for T6 insulin were exploited in a 14-data-set Rietveld refinement to obtain an average crystal structure over the pH range investigated. Only the protein atoms of the known structure with PDB code 2a3g were employed in our starting model. A novel approach for refining protein structures using powder diffraction data is presented. In this approach, each amino acid is represented by a flexible rigid body (FRB). The FRB model requires a significantly smaller number of refinable parameters and restraints than a fully free-atom refinement. A total of 1542 stereochemical restraints were imposed in order to refine the positions of 800 protein atoms, two Zn atoms and 44 water molecules in the asymmetric unit using experimental data in the resolution range 18.2-2.7 Å for all profiles.
- Department of Biology, Section of Genetics, Cell Biology and Development, University of Patras, GR-26500 Patras, Greece. imargiola@upatras.gr
Organizational Affiliation: