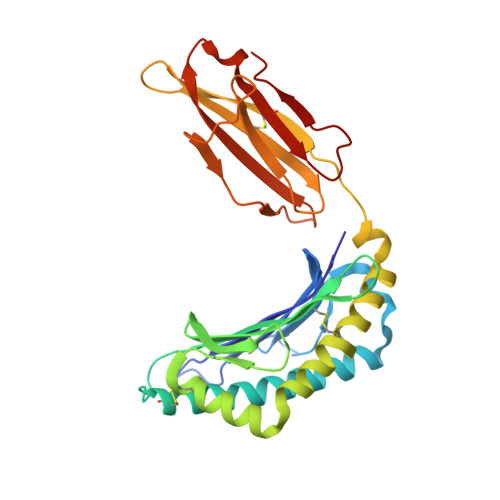
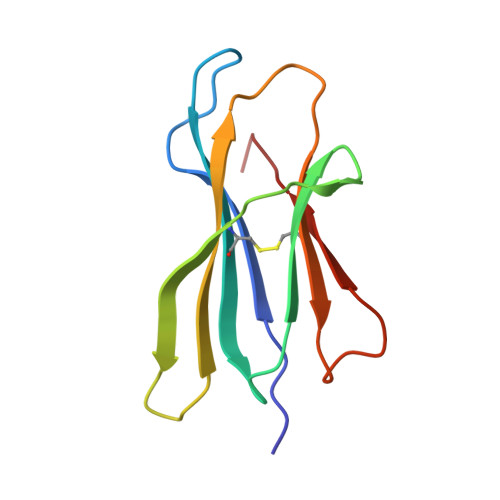
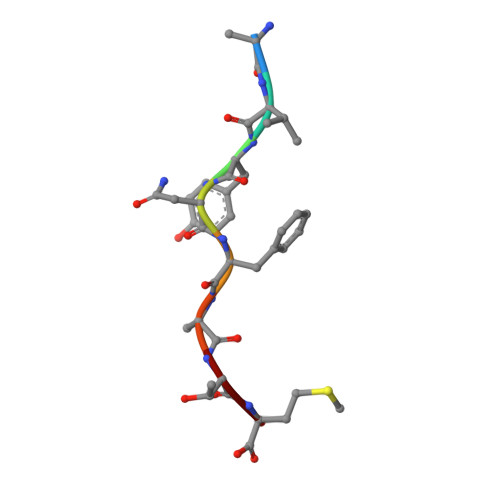
Peptide-independent stabilization of MHC class I molecules breaches cellular quality control.
Hein, Z., Uchtenhagen, H., Abualrous, E.T., Saini, S.K., Janen, L., Van Hateren, A., Wiek, C., Hanenberg, H., Momburg, F., Achour, A., Elliott, T., Springer, S., Boulanger, D.(2014) J Cell Sci 127: 2885-2897
- PubMed: 24806963
- DOI: https://doi.org/10.1242/jcs.145334
- Primary Citation of Related Structures:
4HS3 - PubMed Abstract:
The intracellular trafficking of major histocompatibility complex class I (MHC-I) proteins is directed by three quality control mechanisms that test for their structural integrity, which is correlated to the binding of high-affinity antigenic peptide ligands. To investigate which molecular features of MHC-I these quality control mechanisms detect, we have followed the hypothesis that suboptimally loaded MHC-I molecules are characterized by their conformational mobility in the F-pocket region of the peptide-binding site. We have created a novel variant of an MHC-I protein, K(b)-Y84C, in which two α-helices in this region are linked by a disulfide bond that mimics the conformational and dynamic effects of bound high-affinity peptide. K(b)-Y84C shows a remarkable increase in the binding affinity to its light chain, beta-2 microglobulin (β2m), and bypasses all three cellular quality control steps. Our data demonstrate (1) that coupling between peptide and β2m binding to the MHC-I heavy chain is mediated by conformational dynamics; (2) that the folded conformation of MHC-I, supported by β2m, plays a decisive role in passing the ER-to-cell-surface transport quality controls; and (3) that β2m association is also tested by the cell surface quality control that leads to MHC-I endocytosis.
- Molecular Life Science Center, Jacobs University Bremen, 28759 Bremen, Germany.
Organizational Affiliation: