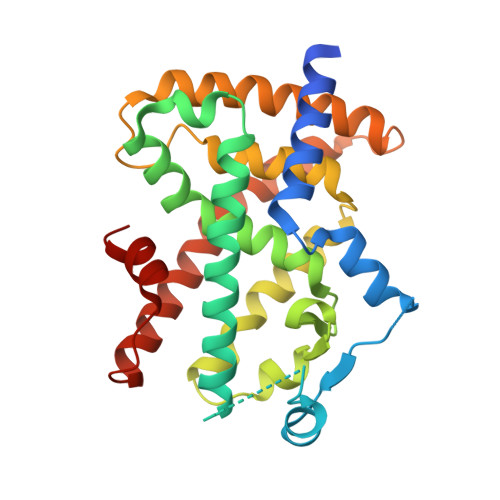
Design, synthesis, and evaluation of imidazo[4,5-c]pyridin-4-one derivatives with dual activity at angiotensin II type 1 receptor and peroxisome proliferator-activated receptor-gamma
Casimiro-Garcia, A., Heemstra, R.J., Bigge, C.F., Chen, J., Ciske, F.A., Davis, J.A., Ellis, T., Esmaeil, N., Flynn, D., Han, S., Jalaie, M., Ohren, J.F., Powell, N.A.(2013) Bioorg Med Chem Lett 23: 767-772
- PubMed: 23265881
- DOI: https://doi.org/10.1016/j.bmcl.2012.11.088
- Primary Citation of Related Structures:
4HEE - PubMed Abstract:
Identification of a series of imidazo[4,5-c]pyridin-4-one derivatives that act as dual angiotensin II type 1 (AT1) receptor antagonists and peroxisome proliferator-activated receptor-γ (PPARγ) partial agonists is described. Starting from a known AT1 antagonist template, conformational restriction was introduced by incorporation of an indane ring that when combined with appropriate substitution at the imidazo[4,5-c]pyridin-4-one provided novel series 5 possessing the desired dual activity. The mode of interaction of this series with PPARγ was corroborated through the X-ray crystal structure of 12b bound to the human PPARγ ligand binding domain. Modulation of activity at both receptors through substitution at the pyridone nitrogen led to the identification of potent dual AT1 antagonists/PPARγ partial agonists. Among them, 21b was identified possessing potent dual pharmacology (AT1 IC(50) = 7 nM; PPARγ EC(50) = 295 nM, 27% max) and good ADME properties.
- Worldwide Medicinal Chemistry, Pfizer Worldwide Research and Development, 200 Cambridgepark Drive, Cambridge, MA 02140, USA. agustin.casimiro-garcia@pfizer.com
Organizational Affiliation: