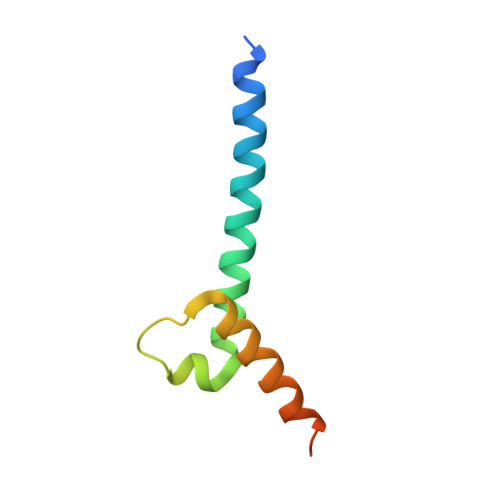
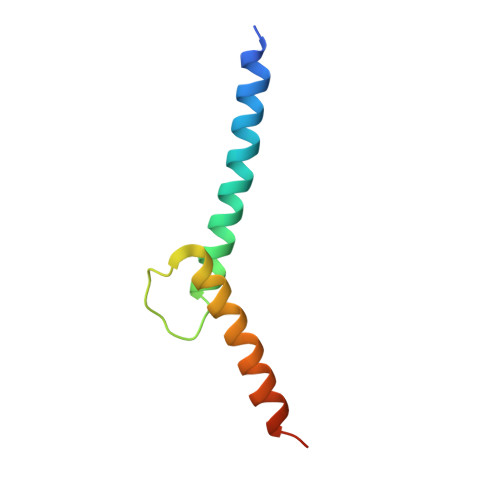
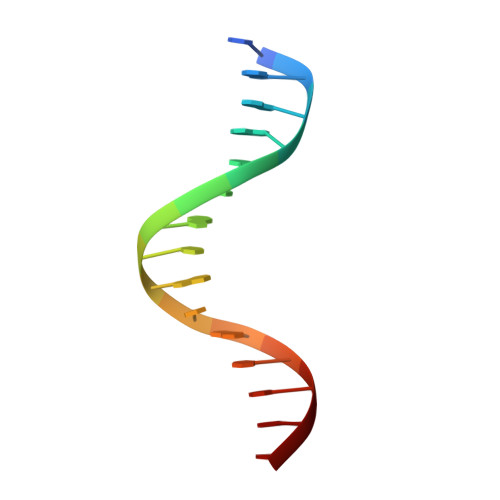
Intermolecular recognition revealed by the complex structure of human CLOCK-BMAL1 basic helix-loop-helix domains with E-box DNA.
Wang, Z., Wu, Y., Li, L., Su, X.D.(2013) Cell Res 23: 213-224
- PubMed: 23229515
- DOI: https://doi.org/10.1038/cr.2012.170
- Primary Citation of Related Structures:
4H10 - PubMed Abstract:
CLOCK (circadian locomotor output cycles kaput) and BMAL1 (brain and muscle ARNT-like 1) are both transcription factors of the circadian core loop in mammals. Recently published mouse CLOCK-BMAL1 bHLH (basic helix-loop-helix)-PAS (period-ARNT-single-minded) complex structure sheds light on the mechanism for heterodimer formation, but the structural details of the protein-DNA recognition mechanisms remain elusive. Here we have elucidated the crystal structure of human CLOCK-BMAL1 bHLH domains bound to a canonical E-box DNA. We demonstrate that CLOCK and BMAL1 bHLH domains can be mutually selected, and that hydrogen-bonding networks mediate their E-box recognition. We identified a hydrophobic contact between BMAL1 Ile80 and a flanking thymine nucleotide, suggesting that CLOCK-BMAL1 actually reads 7-bp DNA and not the previously believed 6-bp DNA. To find potential non-canonical E-boxes that could be recognized by CLOCK-BMAL1, we constructed systematic single-nucleotide mutations on the E-box and measured their relevant affinities. We defined two non-canonical E-box patterns with high affinities, AACGTGA and CATGTGA, in which the flanking A7-T7' base pair is indispensable for recognition. These results will help us to identify functional CLOCK-BMAL1-binding sites in vivo and to search for clock-controlled genes. Furthermore, we assessed the inhibitory role of potential phosphorylation sites in bHLH regions. We found that the phospho-mimicking mutation on BMAL1 Ser78 could efficiently block DNA binding as well as abolish normal circadian oscillation in cells. We propose that BMAL1 Ser78 should be a key residue mediating input signal-regulated transcriptional inhibition for external cues to entrain the circadian clock by kinase cascade.
- State Key Laboratory of Protein and Plant Gene Research, and Biodynamic Optical Imaging Center (BIOPIC), Peking University, Beijing 100871, China.
Organizational Affiliation: