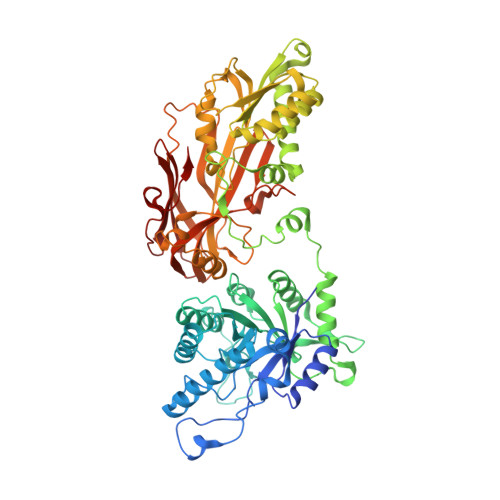
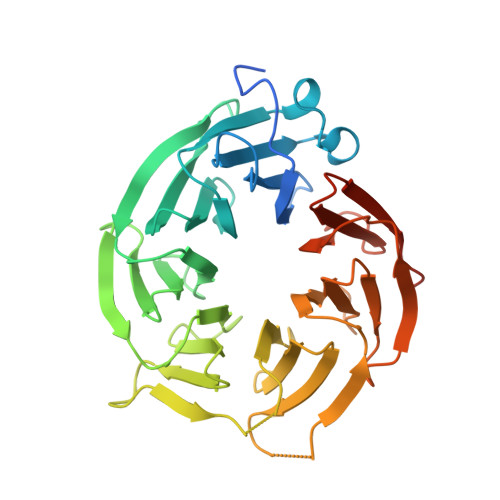
Crystal structure of the human PRMT5:MEP50 complex.
Antonysamy, S., Bonday, Z., Campbell, R.M., Doyle, B., Druzina, Z., Gheyi, T., Han, B., Jungheim, L.N., Qian, Y., Rauch, C., Russell, M., Sauder, J.M., Wasserman, S.R., Weichert, K., Willard, F.S., Zhang, A., Emtage, S.(2012) Proc Natl Acad Sci U S A 109: 17960-17965
- PubMed: 23071334
- DOI: https://doi.org/10.1073/pnas.1209814109
- Primary Citation of Related Structures:
4GQB - PubMed Abstract:
Protein arginine methyltransferases (PRMTs) play important roles in several cellular processes, including signaling, gene regulation, and transport of proteins and nucleic acids, to impact growth, differentiation, proliferation, and development. PRMT5 symmetrically di-methylates the two-terminal ω-guanidino nitrogens of arginine residues on substrate proteins. PRMT5 acts as part of a multimeric complex in concert with a variety of partner proteins that regulate its function and specificity. A core component of these complexes is the WD40 protein MEP50/WDR77/p44, which mediates interactions with binding partners and substrates. We have determined the crystal structure of human PRMT5 in complex with MEP50 (methylosome protein 50), bound to an S-adenosylmethionine analog and a peptide substrate derived from histone H4. The structure of the surprising hetero-octameric complex reveals the close interaction between the seven-bladed β-propeller MEP50 and the N-terminal domain of PRMT5, and delineates the structural elements of substrate recognition.
- Lilly Biotechnology Center, Eli Lilly and Company, San Diego, CA 92121, USA.
Organizational Affiliation: