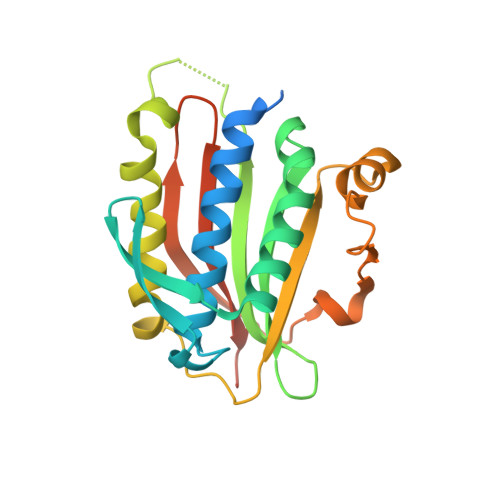
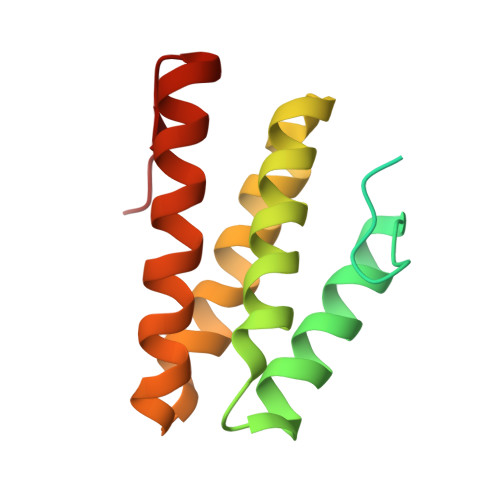
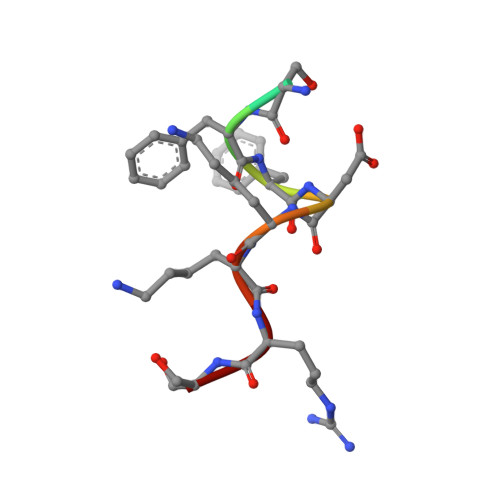
Structural insights into the assembly of human translesion polymerase complexes
Xie, W., Yang, X., Xu, M., Jiang, T.(2012) Protein Cell 3: 864-874
- PubMed: 23143872
- DOI: https://doi.org/10.1007/s13238-012-2102-x
- Primary Citation of Related Structures:
4GK0, 4GK5 - PubMed Abstract:
In addition to DNA repair pathways, cells utilize translesion DNA synthesis (TLS) to bypass DNA lesions during replication. During TLS, Y-family DNA polymerase (Polη, Polκ, Polı and Rev1) inserts specific nucleotide opposite preferred DNA lesions, and then Polζ consisting of two subunits, Rev3 and Rev7, carries out primer extension. Here, we report the complex structures of Rev3-Rev7-Rev1(CTD) and Rev3-Rev7-Rev1(CTD)-Polκ(RIR). These two structures demonstrate that Rev1(CTD) contains separate binding sites for Polκ and Rev7. Our BIAcore experiments provide additional support for the notion that the interaction between Rev3 and Rev7 increases the affinity of Rev7 and Rev1. We also verified through FRET experiment that Rev1, Rev3, Rev7 and Polκ form a stable quaternary complex in vivo, thereby suggesting an efficient switching mechanism where the "inserter" polymerase can be immediately replaced by an "extender" polymerase within the same quaternary complex.
- National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Chaoyang District, Beijing, China.
Organizational Affiliation: