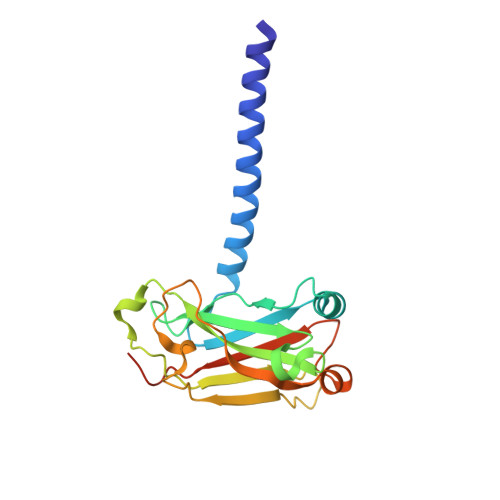
Single Amino Acid Substitutions Confer the Antiviral Activity of the TRAF3 Adaptor Protein onto TRAF5
Zhang, P., Reichardt, A., Liang, H., Aliyari, R., Cheng, D., Wang, Y., Xu, F., Cheng, G., Liu, Y.(2012) Sci Signal 5: ra81-ra81
- PubMed: 23150880
- DOI: https://doi.org/10.1126/scisignal.2003152
- Primary Citation of Related Structures:
4GHU, 4GJH - PubMed Abstract:
The TRAF [tumor necrosis factor receptor-associated factor] family of cytoplasmic adaptor proteins link cell-surface receptors to intracellular signaling pathways that regulate innate and adaptive immune responses. In response to activation of RIG-I (retinoic acid-inducible gene I), a component of a pattern recognition receptor that detects viruses, TRAF3 binds to the adaptor protein Cardif [caspase activation and recruitment domain (CARD) adaptor-inducing interferon-β (IFN-β)], leading to induction of type I IFNs. We report the crystal structures of the TRAF domain of TRAF5 and that of TRAF3 bound to a peptide from the TRAF-interacting motif of Cardif. By comparing these structures, we identified two residues located near the Cardif binding pocket in TRAF3 (Tyr(440) and Phe(473)) that potentially contributed to Cardif recognition. In vitro and cellular experiments showed that forms of TRAF5 with mutation of the corresponding residues to those of TRAF3 had TRAF3-like antiviral activity. Our results provide a structural basis for the critical role of TRAF3 in activating RIG-I-mediated IFN production.
- State Key Laboratory of Biomacromolecules, CAS Key Laboratory of Infection and Immunity, Institute of Biophysics, Chinese Academy of Science, Beijing 100101, China.
Organizational Affiliation: