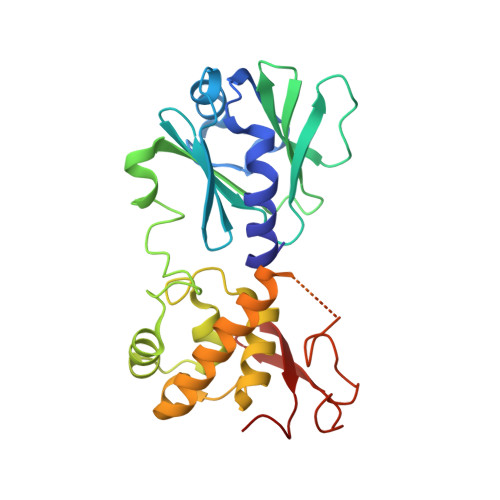
Structural and Biochemical Analysis of DNA Helix Invasion by the Bacterial 8-Oxoguanine DNA Glycosylase MutM.
Sung, R.J., Zhang, M., Qi, Y., Verdine, G.L.(2013) J Biological Chem 288: 10012-10023
- PubMed: 23404556
- DOI: https://doi.org/10.1074/jbc.M112.415612
- Primary Citation of Related Structures:
4G4N, 4G4O, 4G4Q, 4G4R - PubMed Abstract:
MutM is a bacterial DNA glycosylase that serves as the first line of defense against the highly mutagenic 8-oxoguanine (oxoG) lesion, catalyzing glycosidic bond cleavage of oxoG to initiate base excision DNA repair. Previous work has shown that MutM actively interrogates DNA for the presence of an intrahelical oxoG lesion. This interrogation process involves significant buckling and bending of the DNA to promote extrusion of oxoG from the duplex. Structural snapshots have revealed several different highly conserved residues that are prominently inserted into the duplex in the vicinity of the target oxoG before and after base extrusion has occurred. However, the roles of these helix-invading residues during the lesion recognition and base extrusion process remain unclear. In this study, we set out to probe the function of residues Phe(114) and Met(77) in oxoG recognition and repair. Here we report a detailed biochemical and structural characterization of MutM variants containing either a F114A or M77A mutation, both of which showed significant decreases in the efficiency of oxoG repair. These data reveal that Met(77) plays an important role in stabilizing the lesion-extruded conformation of the DNA. Phe(114), on the other hand, appears to destabilize the intrahelical state of the oxoG lesion, primarily by buckling the target base pair. We report the observation of a completely unexpected interaction state, in which the target base pair is ruptured but remains fully intrahelical; this structure vividly illustrates the disruptive influence of MutM on the target base pair.
- Department of Molecular and Cellular Biology, Harvard University, Cambridge, Massachusetts 02138; Department of Stem Cell and Regenerative Biology, Harvard University, Cambridge, Massachusetts 02138.
Organizational Affiliation: