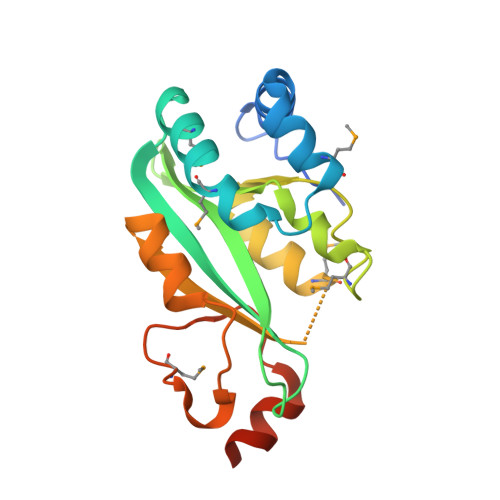
Structure of the catalytic domain of the Salmonella virulence factor SseI.
Bhaskaran, S.S., Stebbins, C.E.(2012) Acta Crystallogr D Biol Crystallogr 68: 1613-1621
- PubMed: 23151626
- DOI: https://doi.org/10.1107/S0907444912039042
- Primary Citation of Related Structures:
4G29, 4G2B - PubMed Abstract:
SseI is secreted into host cells by Salmonella and contributes to the establishment of systemic infections. The crystal structure of the C-terminal domain of SseI has been solved to 1.70 Å resolution, revealing it to be a member of the cysteine protease superfamily with a catalytic triad consisting of Cys178, His216 and Asp231 that is critical to its virulence activities. Structure-based analysis revealed that SseI is likely to possess either acyl hydrolase or acyltransferase activity, placing this virulence factor in the rapidly growing class of enzymes of this family utilized by bacterial pathogens inside eukaryotic cells.
- Laboratory of Structural Microbiology, The Rockefeller University, New York, NY 10065, USA.
Organizational Affiliation: