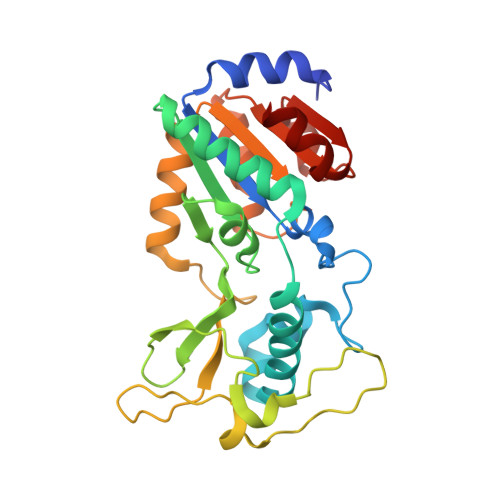
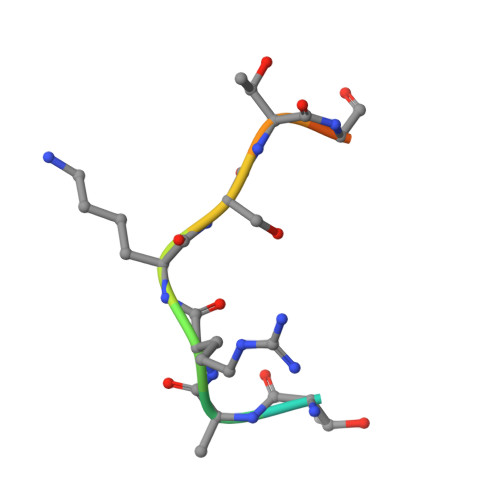
The Bicyclic Intermediate Structure Provides Insights into the Desuccinylation Mechanism of Human Sirtuin 5 (SIRT5)
Zhou, Y., Zhang, H., He, B., Du, J., Lin, H., Cerione, R.A., Hao, Q.(2012) J Biological Chem 287: 28307-28314
- PubMed: 22767592
- DOI: https://doi.org/10.1074/jbc.M112.384511
- Primary Citation of Related Structures:
4F4U, 4F56 - PubMed Abstract:
Sirtuins are pivotal regulators in various cellular processes, including transcription, DNA repair, genome stability, and energy metabolism. Their functions have been generally attributed to NAD-dependent deacetylase activity. However, human SIRT5 (sirtuin 5), which has been reported to exhibit little deacetylase activity, was recently identified as an NAD-dependent demalonylase and desuccinylase. Biochemical studies suggested that the mechanism of SIRT5-catalyzed demalonylation and desuccinylation is similar to that of deacetylation catalyzed by other sirtuins. Previously, we solved the crystal structure of a SIRT5-succinyl-lysine peptide-NAD complex. Here, we present two more structures: a binary complex of SIRT5 with an H3K9 succinyl peptide and a binary complex of SIRT5 with a bicyclic intermediate obtained by incubating SIRT5-H3K9 thiosuccinyl peptide co-crystals with NAD. To our knowledge, this represents the first bicyclic intermediate for a sirtuin-catalyzed deacylation reaction that has been captured in a crystal structure, thus providing unique insights into the reaction mechanism. The structural information should benefit the design of specific inhibitors for SIRT5 and help in exploring the therapeutic potential of targeting sirtuins for treating human diseases.
- MacCHESS, Cornell High Energy Synchrotron Source, Cornell University, Ithaca, New York 14853, USA.
Organizational Affiliation: