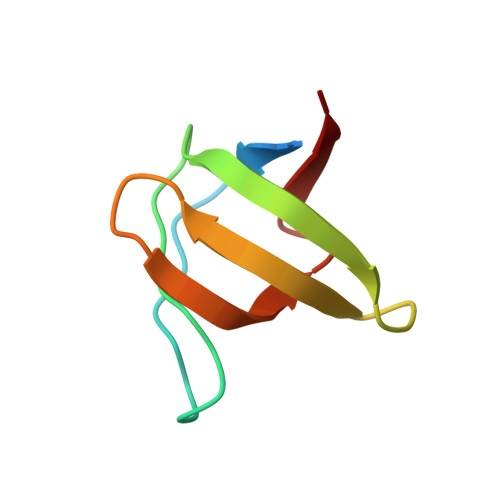
Identification of Xin-repeat proteins as novel ligands of the SH3 domains of nebulin and nebulette and analysis of their interaction during myofibril formation and remodeling.
Eulitz, S., Sauer, F., Pelissier, M.C., Boisguerin, P., Molt, S., Schuld, J., Orfanos, Z., Kley, R.A., Volkmer, R., Wilmanns, M., Kirfel, G., van der Ven, P.F., Furst, D.O.(2013) Mol Biol Cell 24: 3215-3226
- PubMed: 23985323
- DOI: https://doi.org/10.1091/mbc.E13-04-0202
- Primary Citation of Related Structures:
4F14 - PubMed Abstract:
The Xin actin-binding repeat-containing proteins Xin and XIRP2 are exclusively expressed in striated muscle cells, where they are believed to play an important role in development. In adult muscle, both proteins are concentrated at attachment sites of myofibrils to the membrane. In contrast, during development they are localized to immature myofibrils together with their binding partner, filamin C, indicating an involvement of both proteins in myofibril assembly. We identify the SH3 domains of nebulin and nebulette as novel ligands of proline-rich regions of Xin and XIRP2. Precise binding motifs are mapped and shown to bind both SH3 domains with micromolar affinity. Cocrystallization of the nebulette SH3 domain with the interacting XIRP2 peptide PPPTLPKPKLPKH reveals selective interactions that conform to class II SH3 domain-binding peptides. Bimolecular fluorescence complementation experiments in cultured muscle cells indicate a temporally restricted interaction of Xin-repeat proteins with nebulin/nebulette during early stages of myofibril development that is lost upon further maturation. In mature myofibrils, this interaction is limited to longitudinally oriented structures associated with myofibril development and remodeling. These data provide new insights into the role of Xin actin-binding repeat-containing proteins (together with their interaction partners) in myofibril assembly and after muscle damage.
- Institute for Cell Biology, University of Bonn, D-53121 Bonn, Germany European Molecular Biology Laboratory-Hamburg/Deutsches Elektronen-Synchrotron, D-22603 Hamburg, Germany Department of Medicinal Immunology, Charité-University Medicine Berlin, D-13353 Berlin, Germany Department of Neurology, Neuromuscular Center Ruhrgebiet, University Hospital Bergmannsheil, Ruhr-University Bochum, D-44789 Bochum, Germany.
Organizational Affiliation: