Structural studies of DnaK in complex with proline rich antimicrobial peptides reveal two different peptide binding modes
Zahn, M., Straeter, N.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Chaperone protein DnaK | 219 | Escherichia coli K-12 | Mutation(s): 0 Gene Names: b0014, dnaK, groP, grpF, JW0013, seg | 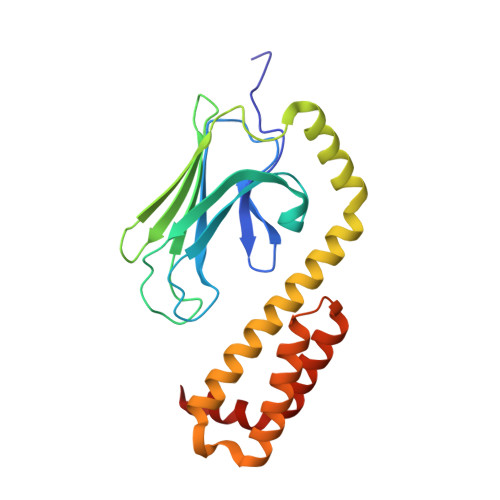 | |
UniProt | |||||
Find proteins for P0A6Y8 (Escherichia coli (strain K12)) Explore P0A6Y8 Go to UniProtKB: P0A6Y8 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0A6Y8 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SO4 Query on SO4 | C [auth A] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 94.437 | α = 90 |
| b = 116.905 | β = 90 |
| c = 36.994 | γ = 90 |
| Software Name | Purpose |
|---|---|
| MAR345 | data collection |
| REFMAC | refinement |
| XDS | data reduction |
| SCALA | data scaling |
| REFMAC | phasing |