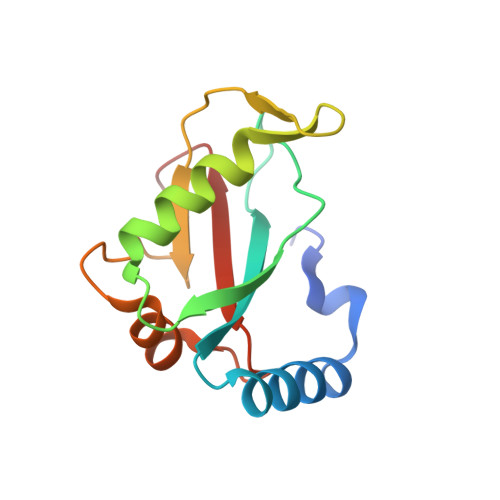
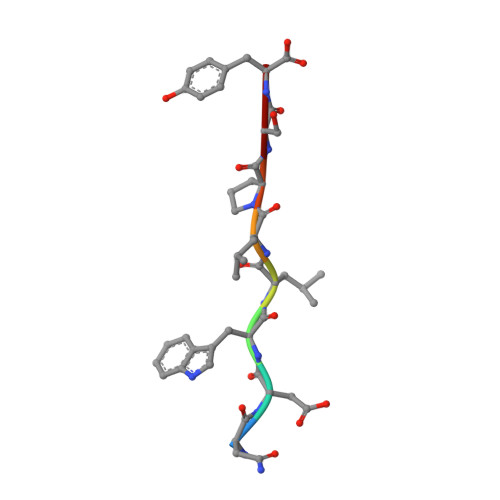
Structural characterization and inhibition of the Plasmodium Atg8-Atg3 interaction.
Hain, A.U., Weltzer, R.R., Hammond, H., Jayabalasingham, B., Dinglasan, R.R., Graham, D.R., Colquhoun, D.R., Coppens, I., Bosch, J.(2012) J Struct Biol 180: 551-562
- PubMed: 22982544
- DOI: https://doi.org/10.1016/j.jsb.2012.09.001
- Primary Citation of Related Structures:
4EOY - PubMed Abstract:
The autophagy-related proteins are thought to serve multiple functions in Plasmodium and are considered essential to parasite survival and development. We have studied two key interacting proteins, Atg8 and Atg3, of the autophagy pathway in Plasmodium falciparum. These proteins are vital for the formation and elongation of the autophagosome and essential to the process of macroautophagy. Autophagy may be required for conversion of the sporozoite into erythrocytic-infective merozoites and may be crucial for other functions during asexual blood stages. Here we describe the identification of an Atg8 family interacting motif (AIM) in Plasmodium Atg3, which binds Plasmodium Atg8. We determined the co-crystal structure of PfAtg8 with a short Atg3¹⁰³⁻¹¹⁰ peptide, corresponding to this motif, to 2.2 Å resolution. Our in vitro interaction studies are in agreement with our X-ray crystal structure. Furthermore they suggest an important role for a unique Apicomplexan loop absent from human Atg8 homologues. Prevention of the protein-protein interaction of full length PfAtg8 with PfAtg3 was achieved at low micromolar concentrations with a small molecule, 1,2,3-trihydroxybenzene. Together our structural and interaction studies represent a starting point for future antimalarial drug discovery and design for this novel protein-protein interaction.
- Department of Biochemistry and Molecular Biology, Johns Hopkins School of Public Health, Baltimore, MD 21205, USA.
Organizational Affiliation: