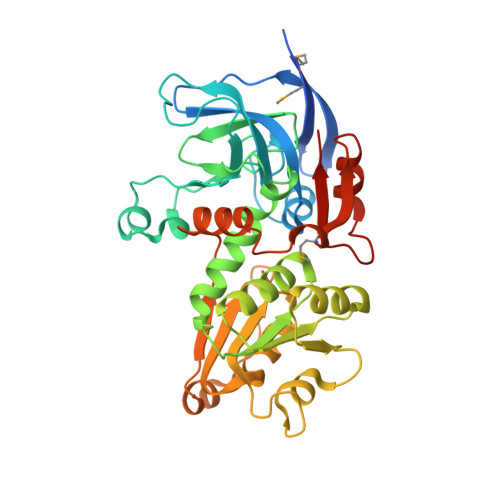
Crystal structure of a putative zinc-binding dehydrogenase (target nysgrc-012003) from sinorhizobium meliloti 1021 bound to NADP
Sampathkumar, P., Banu, N., Bhosle, R., Bonanno, J., Chamala, S., Chowdhury, S., Fiser, A., Gizzi, A., Glenn, A.S., Hammonds, J., Hillerich, B., Khafizov, K., Love, J.D., Matikainen, B., Patskovsky, Y., Seidel, R., Toro, R., Zencheck, W., Almo, S.C.To be published.