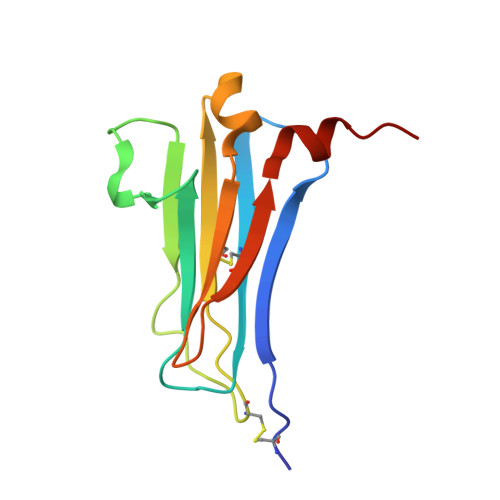
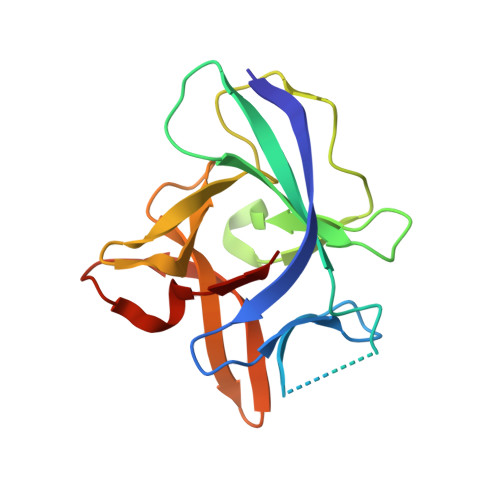
A unique bivalent binding and inhibition mechanism by the yatapoxvirus interleukin 18 binding protein.
Krumm, B., Meng, X., Wang, Z., Xiang, Y., Deng, J.(2012) PLoS Pathog 8: e1002876-e1002876
- PubMed: 22927815
- DOI: https://doi.org/10.1371/journal.ppat.1002876
- Primary Citation of Related Structures:
4EEE, 4EKX - PubMed Abstract:
Interleukin 18 (IL18) is a cytokine that plays an important role in inflammation as well as host defense against microbes. Mammals encode a soluble inhibitor of IL18 termed IL18 binding protein (IL18BP) that modulates IL18 activity through a negative feedback mechanism. Many poxviruses encode homologous IL18BPs, which contribute to virulence. Previous structural and functional studies on IL18 and IL18BPs revealed an essential binding hot spot involving a lysine on IL18 and two aromatic residues on IL18BPs. The aromatic residues are conserved among the very diverse mammalian and poxviruses IL18BPs with the notable exception of yatapoxvirus IL18BPs, which lack a critical phenylalanine residue. To understand the mechanism by which yatapoxvirus IL18BPs neutralize IL18, we solved the crystal structure of the Yaba-Like Disease Virus (YLDV) IL18BP and IL18 complex at 1.75 Å resolution. YLDV-IL18BP forms a disulfide bonded homo-dimer engaging IL18 in a 2∶2 stoichiometry, in contrast to the 1∶1 complex of ectromelia virus (ECTV) IL18BP and IL18. Disruption of the dimer interface resulted in a functional monomer, however with a 3-fold decrease in binding affinity. The overall architecture of the YLDV-IL18BP:IL18 complex is similar to that observed in the ECTV-IL18BP:IL18 complex, despite lacking the critical lysine-phenylalanine interaction. Through structural and mutagenesis studies, contact residues that are unique to the YLDV-IL18BP:IL18 binding interface were identified, including Q67, P116 of YLDV-IL18BP and Y1, S105 and D110 of IL18. Overall, our studies show that YLDV-IL18BP is unique among the diverse family of mammalian and poxvirus IL-18BPs in that it uses a bivalent binding mode and a unique set of interacting residues for binding IL18. However, despite this extensive divergence, YLDV-IL18BP binds to the same surface of IL18 used by other IL18BPs, suggesting that all IL18BPs use a conserved inhibitory mechanism by blocking a putative receptor-binding site on IL18.
- Department of Biochemistry and Molecular Biology, Oklahoma State University, Stillwater, Oklahoma, United States of America.
Organizational Affiliation: