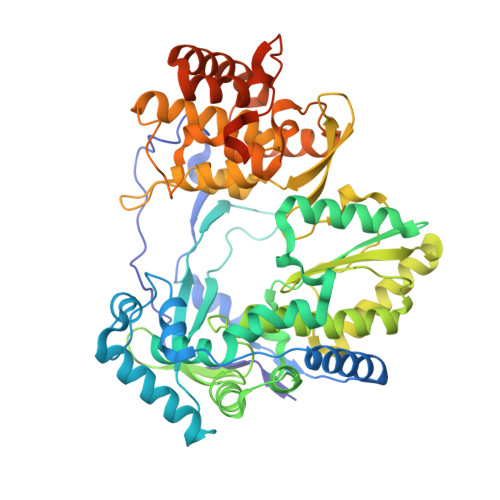
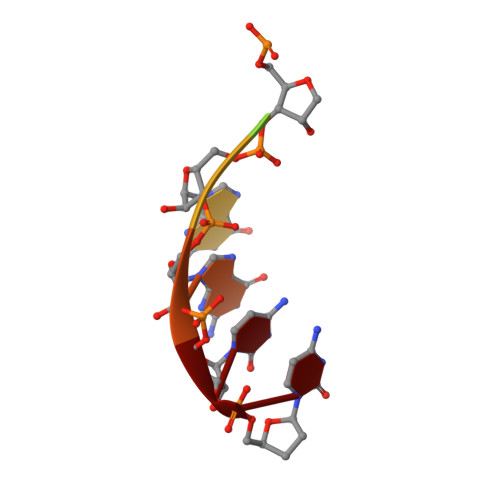
Structure of hepatitis C virus polymerase in complex with primer-template RNA.
Mosley, R.T., Edwards, T.E., Murakami, E., Lam, A.M., Grice, R.L., Du, J., Sofia, M.J., Furman, P.A., Otto, M.J.(2012) J Virol 86: 6503-6511
- PubMed: 22496223
- DOI: https://doi.org/10.1128/JVI.00386-12
- Primary Citation of Related Structures:
4E76, 4E78, 4E7A - PubMed Abstract:
The replication of the hepatitis C viral (HCV) genome is accomplished by the NS5B RNA-dependent RNA polymerase (RdRp), for which mechanistic understanding and structure-guided drug design efforts have been hampered by its propensity to crystallize in a closed, polymerization-incompetent state. The removal of an autoinhibitory β-hairpin loop from genotype 2a HCV NS5B increases de novo RNA synthesis by >100-fold, promotes RNA binding, and facilitated the determination of the first crystallographic structures of HCV polymerase in complex with RNA primer-template pairs. These crystal structures demonstrate the structural realignment required for primer-template recognition and elongation, provide new insights into HCV RNA synthesis at the molecular level, and may prove useful in the structure-based design of novel antiviral compounds. Additionally, our approach for obtaining the RNA primer-template-bound structure of HCV polymerase may be generally applicable to solving RNA-bound complexes for other viral RdRps that contain similar regulatory β-hairpin loops, including bovine viral diarrhea virus, dengue virus, and West Nile virus.
- Pharmasset Inc, Princeton, New Jersey, USA.
Organizational Affiliation: