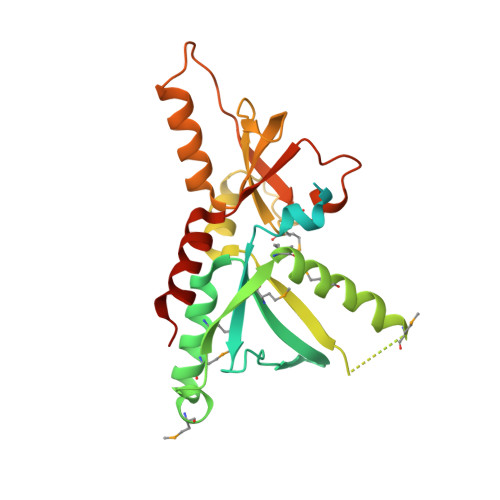
Tic22 is an essential chaperone required for protein import into the apicoplast.
Glaser, S., van Dooren, G.G., Agrawal, S., Brooks, C.F., McFadden, G.I., Striepen, B., Higgins, M.K.(2012) J Biological Chem 287: 39505-39512
- PubMed: 23027875
- DOI: https://doi.org/10.1074/jbc.M112.405100
- Primary Citation of Related Structures:
4E6Z - PubMed Abstract:
Most plastids proteins are post-translationally imported into organelles through multisubunit translocons. The TIC and TOC complexes perform this role in the two membranes of the plant chloroplast and in the inner two membranes of the apicoplasts of the apicomplexan parasites, Toxoplasma gondii and Plasmodium falciparum. Tic22 is a ubiquitous intermembrane translocon component that interacts with translocating proteins. Here, we demonstrate that T. gondii Tic22 is an apicoplast-localized protein, essential for parasite survival and protein import into the apicoplast stroma. The structure of Tic22 from P. falciparum reveals a fold conserved from cyanobacteria to plants, which displays a non-polar groove on each side of the molecule. We show that these grooves allow Tic22 to act as a chaperone. General chaperones are common components of protein translocation systems where they maintain cargo proteins in an unfolded conformation during transit. Such a chaperone had not been identified in the intermembrane space of plastids and we propose that Tic22 fulfills this role.
- Department of Biochemistry, University of Cambridge, Cambridge, CB2 1GA, England.
Organizational Affiliation: