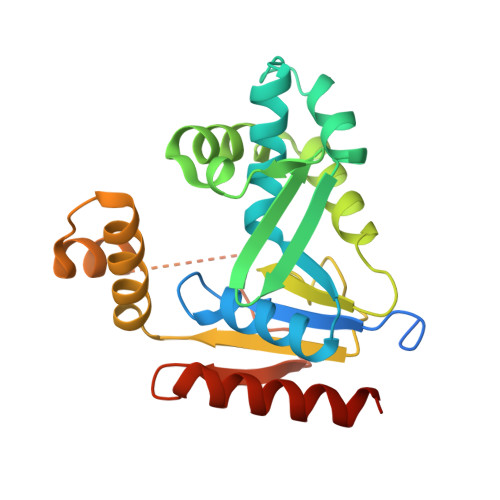
Structure and function of cytidine monophosphate kinase from Yersinia pseudotuberculosis, essential for virulence but not for survival.
Walker, N.J., Clark, E.A., Ford, D.C., Bullifent, H.L., McAlister, E.V., Duffield, M.L., Acharya, K.R., Oyston, P.C.(2012) Open Biol 2: 120142-120142
- PubMed: 23271832
- DOI: https://doi.org/10.1098/rsob.120142
- Primary Citation of Related Structures:
4E22 - PubMed Abstract:
The need for new antibiotics has become pressing in light of the emergence of antibiotic-resistant strains of human pathogens. Yersinia pestis, the causative agent of plague, is a public health threat and also an agent of concern in biodefence. It is a recently emerged clonal derivative of the enteric pathogen Yersinia pseudotuberculosis. Previously, we developed a bioinformatic approach to identify proteins that may be suitable targets for antimicrobial therapy and in particular for the treatment of plague. One such target was cytidine monophosphate (CMP) kinase, which is an essential gene in some organisms. Previously, we had thought CMP kinase was essential for Y. pseudotuberculosis, but by modification of the mutagenesis approach, we report here the production and characterization of a Δcmk mutant. The isogenic mutant had a growth defect relative to the parental strain, and was highly attenuated in mice. We have also elucidated the structure of the CMP kinase to 2.32 Å, and identified three key residues in the active site that are essential for activity of the enzyme. These findings will have implications for the development of novel CMP kinase inhibitors for therapeutic use.
- Biomedical Sciences, Defence Science and Technology Laboratory, Porton Down, Salisbury SP4 0JQ, UK.
Organizational Affiliation: