Conformational plasticity of alpha-catenin revealed by binding interactions with vinculin
Choi, H.-J., Pokutta, S., Bankston, L., Liddington, R., Weis, W.I.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Vinculin | 263 | Gallus gallus | Mutation(s): 0 Gene Names: VCL, VINC1 | 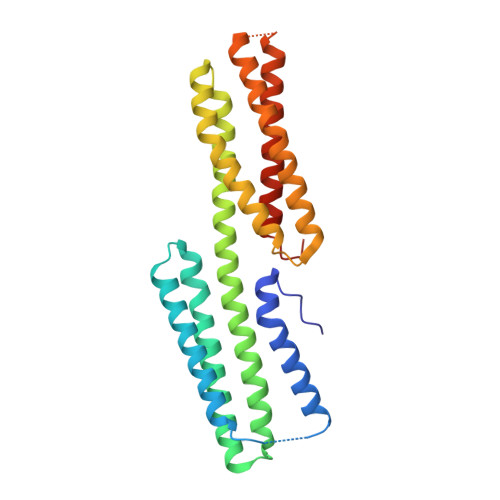 | |
UniProt | |||||
Find proteins for P12003 (Gallus gallus) Explore P12003 Go to UniProtKB: P12003 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P12003 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Catenin alpha-1 | 40 | Mus musculus | Mutation(s): 0 Gene Names: Ctnna1, Catna1 | 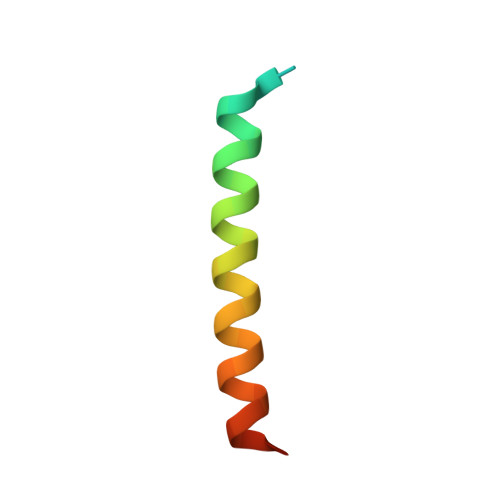 | |
UniProt | |||||
Find proteins for P26231 (Mus musculus) Explore P26231 Go to UniProtKB: P26231 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P26231 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 50.747 | α = 90 |
| b = 55.969 | β = 90 |
| c = 128.015 | γ = 90 |
| Software Name | Purpose |
|---|---|
| SCALEPACK | data scaling |
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |
| HKL-2000 | data collection |
| HKL-2000 | data reduction |
| PHASER | phasing |