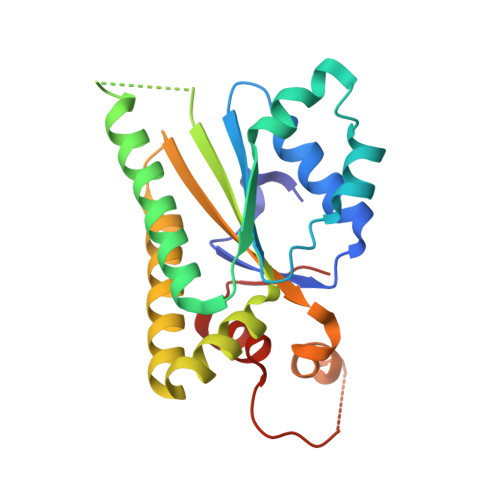
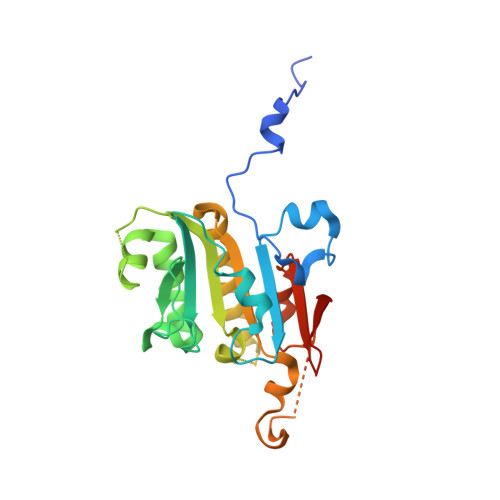
Structural analysis of Shu proteins reveals a DNA binding role essential for resisting damage
Tao, Y., Li, X., Liu, Y., Ruan, J., Qi, S., Niu, L., Teng, M.(2012) J Biological Chem 287: 20231-20239
- PubMed: 22465956
- DOI: https://doi.org/10.1074/jbc.M111.334698
- Primary Citation of Related Structures:
4DT1 - PubMed Abstract:
The yeast Shu complex, consisting of the proteins Shu1, Shu2, Psy3, and Csm2, maintains genomic stability by coupling post-replication repair to homologous recombination. However, a lack of biochemical and structural information on the Shu proteins precludes revealing their precise roles within the pathway. Here, we report on the 1.9-Å crystal structure of the Psy3-Csm2 complex. The crystal structure shows that Psy3 forms a heterodimer with Csm2 mainly through a hydrophobic core. Unexpectedly, Psy3 and Csm2 share a similar architecture that closely resembles the ATPase core domain of Rad51. The L2 loop present in Psy3 and Csm2 is similar to that of Rad51 and confers the DNA binding activity of the Shu complex. As with Rad51, the Shu complex appears to form a nucleoprotein filament by binding nonspecifically to DNA. Structure-based mutagenesis studies have demonstrated that the DNA binding activity of the Shu complex is essential for repair of the methyl methanesulfonate-induced DNA damage. Our findings provide good foundations for the understanding of the Srs2 regulation by the Shu complex.
- Hefei National Laboratory for Physical Sciences at Microscale and School of Life Sciences, University of Science and Technology of China, Hefei, Anhui 230026, China.
Organizational Affiliation: