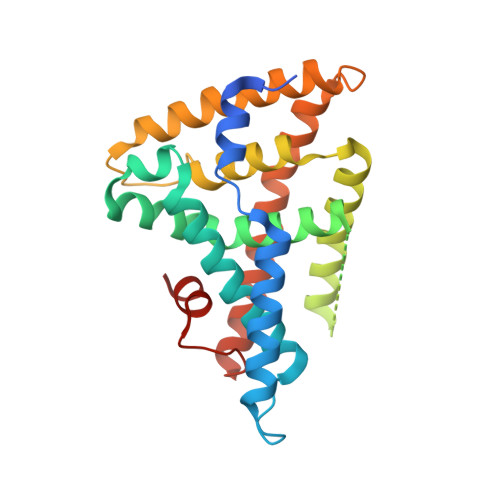
Antidiabetic phospholipid-nuclear receptor complex reveals the mechanism for phospholipid-driven gene regulation.
Musille, P.M., Pathak, M.C., Lauer, J.L., Hudson, W.H., Griffin, P.R., Ortlund, E.A.(2012) Nat Struct Mol Biol 19: 532-537
- PubMed: 22504882
- DOI: https://doi.org/10.1038/nsmb.2279
- Primary Citation of Related Structures:
4DOR, 4DOS - PubMed Abstract:
The human nuclear receptor liver receptor homolog-1 (LRH-1) has an important role in controlling lipid and cholesterol homeostasis and is a potential target for the treatment of diabetes and hepatic diseases. LRH-1 is known to bind phospholipids, but the role of phospholipids in controlling LRH-1 activation remains highly debated. Here we describe the structure of both apo LRH-1 and LRH-1 in complex with the antidiabetic phospholipid dilauroylphosphatidylcholine (DLPC). Together with hydrogen-deuterium exchange MS and functional data, our studies show that DLPC binding is a dynamic process that alters co-regulator selectivity. We show that the lipid-free receptor undergoes previously unrecognized structural fluctuations, allowing it to interact with widely expressed co-repressors. These observations enhance our understanding of LRH-1 regulation and highlight its importance as a new therapeutic target for controlling diabetes.
- Department of Biochemistry, Emory University School of Medicine, Atlanta, GA 30322, USA.
Organizational Affiliation: