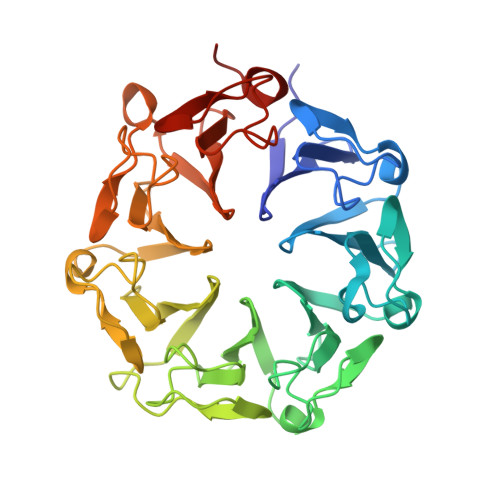
Structural basis of ultraviolet-B perception by UVR8.
Wu, D., Hu, Q., Yan, Z., Chen, W., Yan, C., Huang, X., Zhang, J., Yang, P., Deng, H., Wang, J., Deng, X., Shi, Y.(2012) Nature 484: 214-219
- PubMed: 22388820
- DOI: https://doi.org/10.1038/nature10931
- Primary Citation of Related Structures:
4DNU, 4DNV, 4DNW - PubMed Abstract:
The Arabidopsis thaliana protein UVR8 is a photoreceptor for ultraviolet-B. Upon ultraviolet-B irradiation, UVR8 undergoes an immediate switch from homodimer to monomer, which triggers a signalling pathway for ultraviolet protection. The mechanism by which UVR8 senses ultraviolet-B remains largely unknown. Here we report the crystal structure of UVR8 at 1.8 Å resolution, revealing a symmetric homodimer of seven-bladed β-propeller that is devoid of any external cofactor as the chromophore. Arginine residues that stabilize the homodimeric interface, principally Arg 286 and Arg 338, make elaborate intramolecular cation-π interactions with surrounding tryptophan amino acids. Two of these tryptophans, Trp 285 and Trp 233, collectively serve as the ultraviolet-B chromophore. Our structural and biochemical analyses identify the molecular mechanism for UVR8-mediated ultraviolet-B perception, in which ultraviolet-B radiation results in destabilization of the intramolecular cation-π interactions, causing disruption of the critical intermolecular hydrogen bonds mediated by Arg 286 and Arg 338 and subsequent dissociation of the UVR8 homodimer.
- Tsinghua-Peking Center for Life Sciences, Center for Structural Biology, School of Life Sciences and School of Medicine, Tsinghua University, Beijing 100084, China.
Organizational Affiliation: