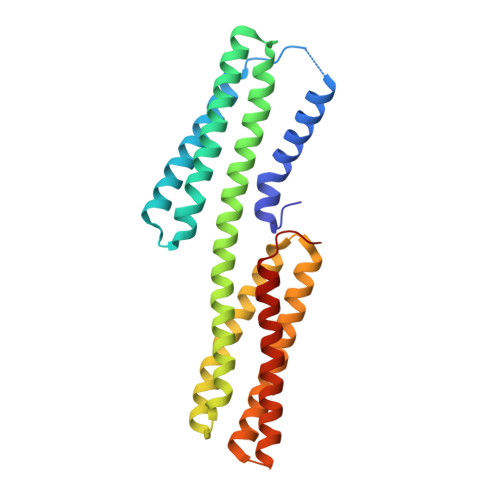
Crystal structure of vinculin in complex with vinculin binding site 50 (VBS50), the integrin binding site 2 (IBS2) of talin.
Yogesha, S.D., Rangarajan, E.S., Vonrhein, C., Bricogne, G., Izard, T.(2012) Protein Sci 21: 583-588
- PubMed: 22334306
- DOI: https://doi.org/10.1002/pro.2041
- Primary Citation of Related Structures:
4DJ9 - PubMed Abstract:
The cytoskeletal protein talin activates integrin receptors by binding of its FERM domain to the cytoplasmic tail of β-integrin. Talin also couples integrins to the actin cytoskeleton, largely by binding to and activating the cytoskeletal protein vinculin, which binds to F-actin through the agency of its five-helix bundle tail (Vt) domain. Talin activates vinculin by means of buried amphipathic α-helices coined vinculin binding sites (VBSs) that reside within numerous four- and five-helix bundle domains that comprise the central talin rod, which are released from their buried locales by means of mechanical tension on the integrin:talin complex. In turn, these VBSs bind to the N-terminal seven-helix bundle (Vh1) domain of vinculin, creating an entirely new helix bundle that severs its head-tail interactions. Interestingly, talin harbors a second integrin binding site coined IBS2 that consists of two five-helix bundle domains that also contain a VBS (VBS50). Here we report the crystal structure of VBS50 in complex with vinculin at 2.3 Å resolution and show that intramolecular interactions of VBS50 within IBS2 are much more extensive versus its interactions with vinculin. Indeed, the IBS2-vinculin interaction only occurs at physiological temperature and the affinity of VBS50 for vinculin is about 30 times less than other VBSs. The data support a model where integrin binding destabilizes IBS2 to allow it to bind to vinculin.
- Department of Cancer Biology, Cell Adhesion Laboratory, The Scripps Research Institute, Jupiter, Florida 33458, USA.
Organizational Affiliation: