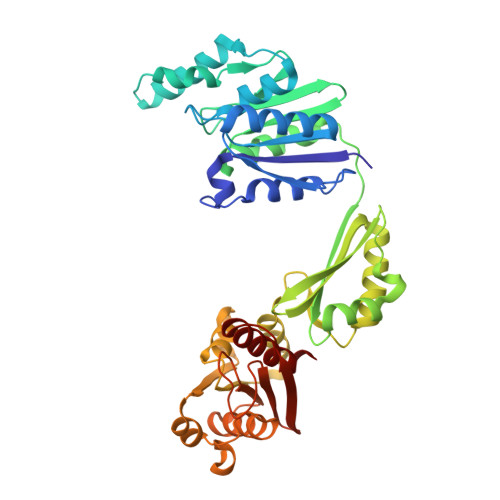
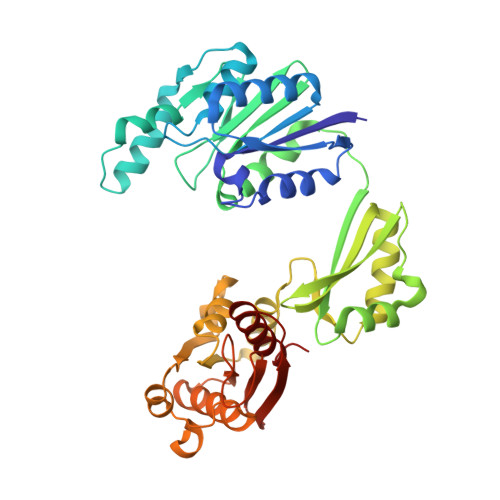
Structure and Mechanism of the Bifunctional Cina Enzyme from Thermus Thermophilus
Karuppiah, V., Thistlethwaite, A., Dajani, R., Warwicker, J., Derrick, J.P.(2014) J Biological Chem 289: 33187
- PubMed: 25313401
- DOI: https://doi.org/10.1074/jbc.M114.608448
- Primary Citation of Related Structures:
4CT8, 4CTA, 4UOC, 4UUW, 4UUX - PubMed Abstract:
CinA is a widely distributed protein in Gram-positive and Gram-negative bacteria. It is associated with natural competence and is proposed to have a function as an enzyme participating in the pyridine nucleotide cycle, which recycles products formed by non-redox uses of NAD. Here we report the determination of the crystal structure of CinA from Thermus thermophilus, in complex with several ligands. CinA was shown to have both nicotinamide mononucleotide deamidase and ADP-ribose pyrophosphatase activities. The crystal structure shows an unusual asymmetric dimer, with three domains for each chain; the C-terminal domain harbors the nicotinamide mononucleotide deamidase activity, and the structure of a complex with the product nicotinate mononucleotide suggests a mechanism for deamidation. The N-terminal domain belongs to the COG1058 family and is associated with the ADP-ribose pyrophosphatase activity. The asymmetry in the CinA dimer arises from two alternative orientations of the COG1058 domains, only one of which forms a contact with the KH-type domain from the other chain, effectively closing the active site into, we propose, a catalytically competent state. Structures of complexes with Mg(2+)/ADP-ribose, Mg(2+)/ATP, and Mg(2+)/AMP suggest a mechanism for the ADP-ribose pyrophosphatase reaction that involves a rotation of the COG1058 domain dimer as part of the reaction cycle, so that each active site oscillates between open and closed forms, thus promoting catalysis.
- From the Faculty of Life Sciences, University of Manchester, Michael Smith Building, Oxford Road, Manchester M13 9PT, United Kingdom.
Organizational Affiliation: