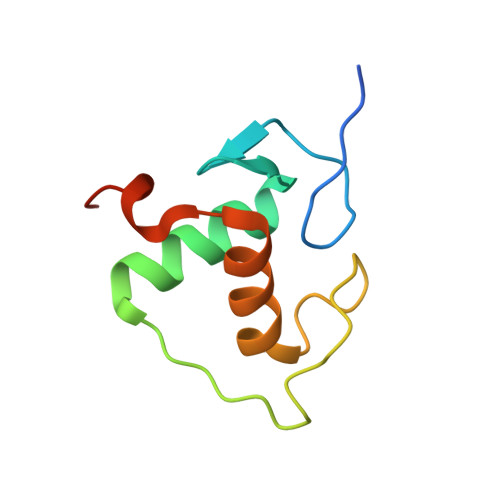
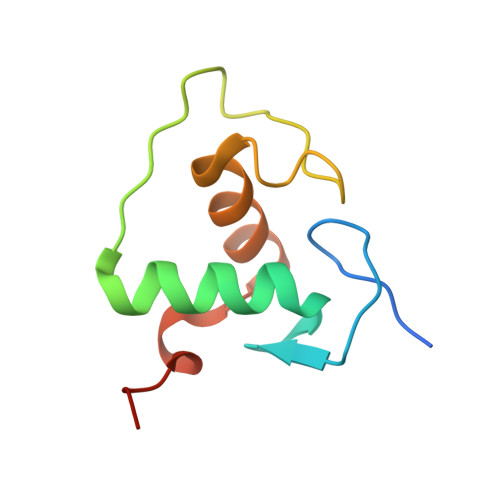
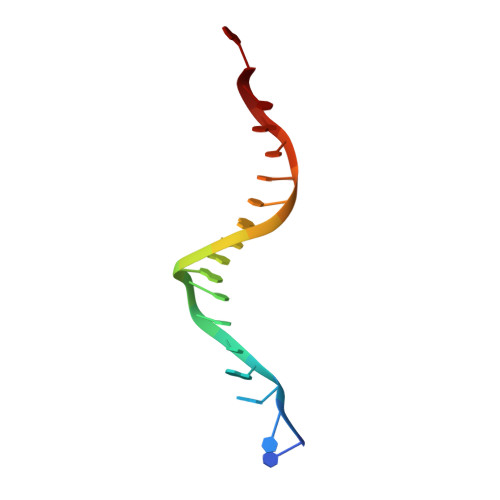
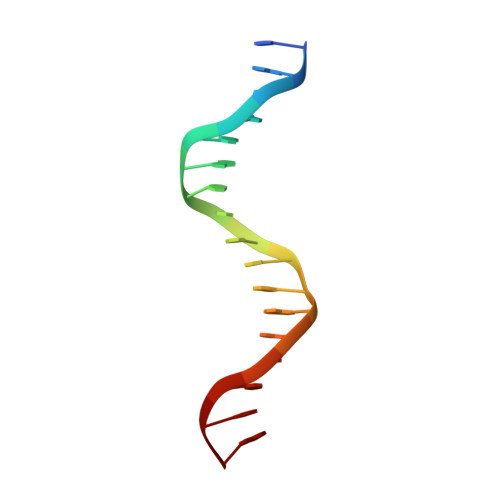
Structural Basis of Natural Promoter Recognition by the Retinoid X Nuclear Receptor.
Osz, J., Mcewen, A.G., Poussin-Courmontagne, P., Moutier, E., Birck, C., Davidson, I., Moras, D., Rochel, N.(2015) Sci Rep 5: 8216
- PubMed: 25645674
- DOI: https://doi.org/10.1038/srep08216
- Primary Citation of Related Structures:
4CN2, 4CN3, 4CN5, 4CN7 - PubMed Abstract:
Retinoid X receptors (RXRs) act as homodimers or heterodimerisation partners of class II nuclear receptors. RXR homo- and heterodimers bind direct repeats of the half-site (A/G)G(G/T)TCA separated by 1 nucleotide (DR1). We present a structural characterization of RXR-DNA binding domain (DBD) homodimers on several natural DR1s and an idealized symmetric DR1. Homodimers displayed asymmetric binding, with critical high-affinity interactions accounting for the 3' positioning of RXR in heterodimers on DR1s. Differing half-site and spacer DNA sequence induce changes in RXR-DBD homodimer conformation notably in the dimerization interface such that natural DR1s are bound with higher affinity than an idealized symmetric DR1. Subtle changes in the consensus DR1 DNA sequence therefore specify binding affinity through altered RXR-DBD-DNA contacts and changes in DBD conformation suggesting a general model whereby preferential half-site recognition determines polarity of heterodimer binding to response elements.
- Department of Integrative Structural Biology, Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC), Institut National de la Santé et de la Recherche Médicale (INSERM) U964/Centre National de la Recherche Scientifique (CNRS) UMR 7104/Université de Strasbourg, 67404 Illkirch, France.
Organizational Affiliation: