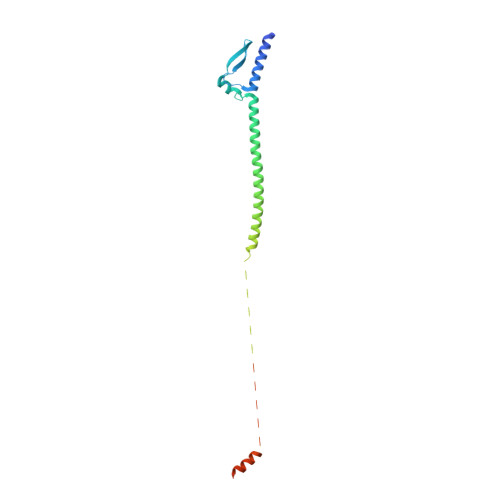
Structure of the Meningococcal Vaccine Antigen Nada and Epitope Mapping of a Bactericidal Antibody.
Malito, E., Biancucci, M., Faleri, A., Ferlenghi, I., Scarselli, M., Maruggi, G., Lo Surdo, P., Veggi, D., Liguori, A., Santini, L., Bertoldi, I., Petracca, R., Marchi, S., Romagnoli, G., Cartocci, E., Vercellino, I., Savino, S., Spraggon, G., Norais, N., Pizza, M., Rappuoli, R., Masignani, V., Bottomley, M.J.(2014) Proc Natl Acad Sci U S A 111: 17128
- PubMed: 25404323
- DOI: https://doi.org/10.1073/pnas.1419686111
- Primary Citation of Related Structures:
4CJD - PubMed Abstract:
Serogroup B Neisseria meningitidis (MenB) is a major cause of severe sepsis and invasive meningococcal disease, which is associated with 5-15% mortality and devastating long-term sequelae. Neisserial adhesin A (NadA), a trimeric autotransporter adhesin (TAA) that acts in adhesion to and invasion of host epithelial cells, is one of the three antigens discovered by genome mining that are part of the MenB vaccine that recently was approved by the European Medicines Agency. Here we present the crystal structure of NadA variant 5 at 2 Å resolution and transmission electron microscopy data for NadA variant 3 that is present in the vaccine. The two variants show similar overall topology with a novel TAA fold predominantly composed of trimeric coiled-coils with three protruding wing-like structures that create an unusual N-terminal head domain. Detailed mapping of the binding site of a bactericidal antibody by hydrogen/deuterium exchange MS shows that a protective conformational epitope is located in the head of NadA. These results provide information that is important for elucidating the biological function and vaccine efficacy of NadA.
- Novartis Vaccines, 53100 Siena, Italy; and enrico.malito@novartis.com rino.rappuoli@novartis.com.
Organizational Affiliation: