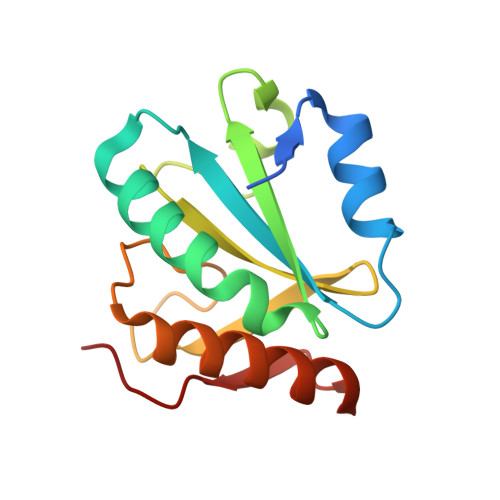
Mutations in the Pqbp1 Gene Prevent its Interaction with the Spliceosomal Protein U5-15Kd.
Mizuguchi, M., Obita, T., Serita, T., Kojima, R., Nabeshima, Y., Okazawa, H.(2014) Nat Commun 5: 3822
- PubMed: 24781215
- DOI: https://doi.org/10.1038/ncomms4822
- Primary Citation of Related Structures:
4BWQ, 4BWS, 4CDO - PubMed Abstract:
A loss-of-function of polyglutamine tract-binding protein 1 (PQBP1) induced by frameshift mutations is believed to cause X-linked mental retardation. However, the mechanism by which structural changes in PQBP1 lead to mental retardation is unknown. Here we present the crystal structure of a C-terminal fragment of PQBP1 in complex with the spliceosomal protein U5-15 kD. The U5-15 kD hydrophobic groove recognizes a YxxPxxVL motif in PQBP1, and mutations within this motif cause a loss-of-function phenotype of PQBP1 in vitro. The YxxPxxVL motif is absent in all PQBP1 frameshift mutants seen in cases of mental retardation. These results suggest a mechanism by which the loss of the YxxPxxVL motif could lead to the functional defects seen in this type of mental retardation.
- 1] Faculty of Pharmaceutical Sciences, University of Toyama; 2630, Sugitani, Toyama 930-0194, Japan [2] Graduate School of Innovative Life Science, University of Toyama; 2630, Sugitani, Toyama 930-0194, Japan [3].
Organizational Affiliation: