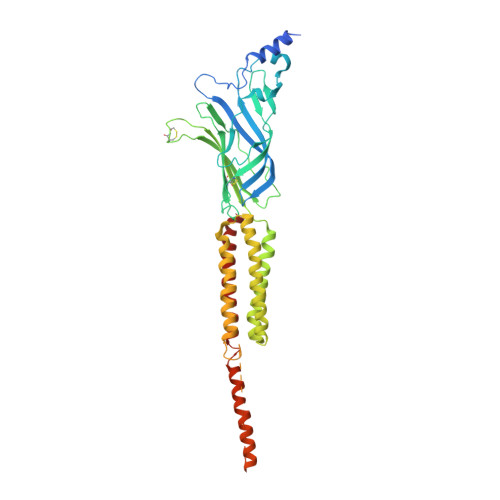
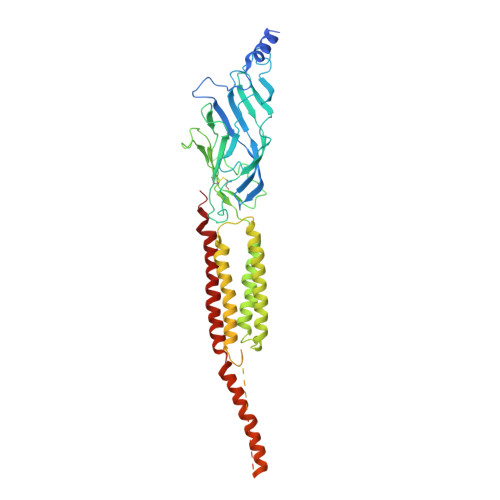
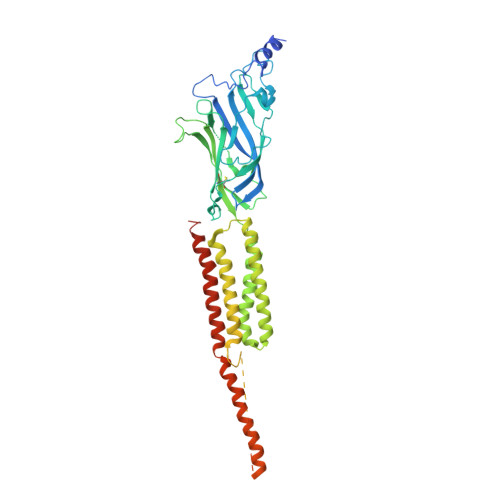
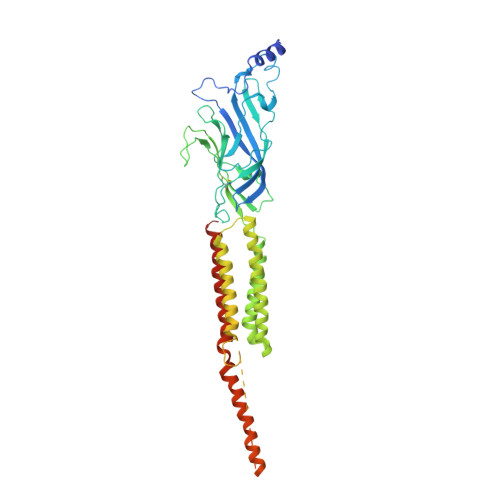
Structure and Superorganization of Acetylcholine Receptor-Rapsyn Complexes.
Zuber, B., Unwin, N.(2013) Proc Natl Acad Sci U S A 110: 10622
- PubMed: 23754381
- DOI: https://doi.org/10.1073/pnas.1301277110
- Primary Citation of Related Structures:
4BOG, 4BOI, 4BON, 4BOO, 4BOR, 4BOT - PubMed Abstract:
The scaffolding protein at the neuromuscular junction, rapsyn, enables clustering of nicotinic acetylcholine receptors in high concentration and is critical for muscle function. Patients with insufficient receptor clustering suffer from muscle weakness. However, the detailed organization of the receptor-rapsyn network is poorly understood: it is unclear whether rapsyn first forms a wide meshwork to which receptors can subsequently dock or whether it only forms short bridges linking receptors together to make a large cluster. Furthermore, the number of rapsyn-binding sites per receptor (a heteropentamer) has been controversial. Here, we show by cryoelectron tomography and subtomogram averaging of Torpedo postsynaptic membrane that receptors are connected by up to three rapsyn bridges, the minimum number required to form a 2D network. Half of the receptors belong to rapsyn-connected groups comprising between two and fourteen receptors. Our results provide a structural basis for explaining the stability and low diffusion of receptors within clusters.
- Laboratory of Experimental Morphology, Institute of Anatomy, University of Bern, CH-3000 Bern 9, Switzerland.
Organizational Affiliation: