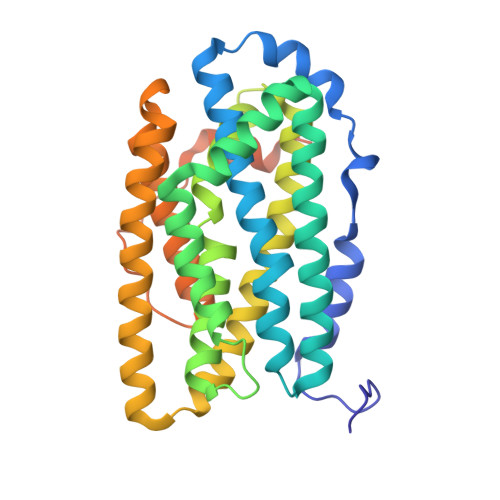
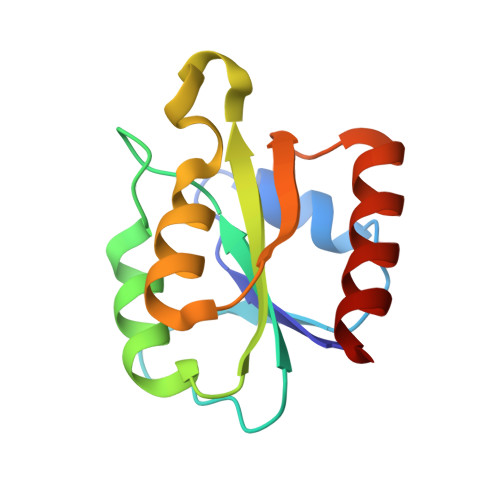
Crystal Structure of Bacillus Cereus Class Ib Ribonucleotide Reductase Di-Iron Nrdf in Complex with Nrdi.
Hammerstad, M., Hersleth, H., Tomter, A.B., Rohr, A.K., Andersson, K.K.(2014) ACS Chem Biol 9: 526
- PubMed: 24295378
- DOI: https://doi.org/10.1021/cb400757h
- Primary Citation of Related Structures:
4BMO, 4BMP, 4BMQ, 4BMR, 4BMT, 4BMU - PubMed Abstract:
Class Ib ribonucleotide reductases (RNRs) use a dimetal-tyrosyl radical (Y•) cofactor in their NrdF (β2) subunit to initiate ribonucleotide reduction in the NrdE (α2) subunit. Contrary to the diferric tyrosyl radical (Fe(III)2-Y•) cofactor, which can self-assemble from Fe(II)2-NrdF and O2, generation of the Mn(III)2-Y• cofactor requires the reduced form of a flavoprotein, NrdIhq, and O2 for its assembly. Here we report the 1.8 Å resolution crystal structure of Bacillus cereus Fe2-NrdF in complex with NrdI. Compared to the previously solved Escherichia coli NrdI-Mn(II)2-NrdF structure, NrdI and NrdF binds similarly in Bacillus cereus through conserved core interactions. This protein-protein association seems to be unaffected by metal ion type bound in the NrdF subunit. The Bacillus cereus Mn(II)2-NrdF and Fe2-NrdF structures, also presented here, show conformational flexibility of residues surrounding the NrdF metal ion site. The movement of one of the metal-coordinating carboxylates is linked to the metal type present at the dimetal site and not associated with NrdI-NrdF binding. This carboxylate conformation seems to be vital for the water network connecting the NrdF dimetal site and the flavin in NrdI. From these observations, we suggest that metal-dependent variations in carboxylate coordination geometries are important for active Y• cofactor generation in class Ib RNRs. Additionally, we show that binding of NrdI to NrdF would structurally interfere with the suggested α2β2 (NrdE-NrdF) holoenzyme formation, suggesting the potential requirement for NrdI dissociation before NrdE-NrdF assembly after NrdI-activation. The mode of interactions between the proteins involved in the class Ib RNR system is, however, not fully resolved.
- Department of Biosciences, University of Oslo , P.O. Box 1066, Blindern, NO-0316 Oslo, Norway.
Organizational Affiliation: