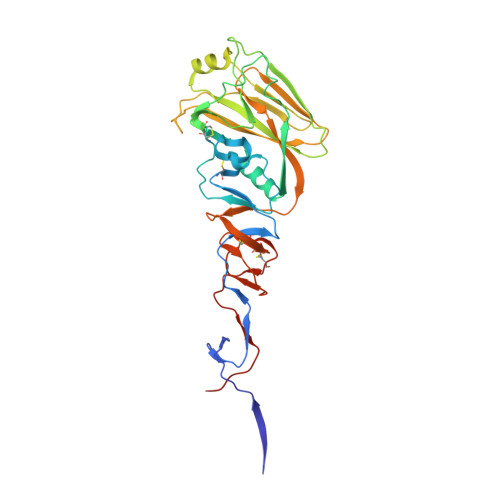
Receptor Binding by a Ferret-Transmissible H5 Avian Influenza Virus.
Xiong, X., Coombs, P., R Martin, S., Liu, J., Xiao, H., Mccauley, J.W., Locher, K., Walker, P.A., Collins, P.J., Kawaoka, Y., Skehel, J.J., Gamblin, S.J.(2013) Nature 497: 392
- PubMed: 23615615
- DOI: https://doi.org/10.1038/nature12144
- Primary Citation of Related Structures:
4BGW, 4BGX, 4BGY, 4BGZ, 4BH0, 4BH1, 4BH2, 4BH3, 4BH4 - PubMed Abstract:
Cell-surface-receptor binding by influenza viruses is a key determinant of their transmissibility, both from avian and animal species to humans as well as from human to human. Highly pathogenic avian H5N1 viruses that are a threat to public health have been observed to acquire affinity for human receptors, and transmissible-mutant-selection experiments have identified a virus that is transmissible in ferrets, the generally accepted experimental model for influenza in humans. Here, our quantitative biophysical measurements of the receptor-binding properties of haemagglutinin (HA) from the transmissible mutant indicate a small increase in affinity for human receptor and a marked decrease in affinity for avian receptor. From analysis of virus and HA binding data we have derived an algorithm that predicts virus avidity from the affinity of individual HA-receptor interactions. It reveals that the transmissible-mutant virus has a 200-fold preference for binding human over avian receptors. The crystal structure of the transmissible-mutant HA in complex with receptor analogues shows that it has acquired the ability to bind human receptor in the same folded-back conformation as seen for HA from the 1918, 1957 (ref. 4), 1968 (ref. 5) and 2009 (ref. 6) pandemic viruses. This binding mode is substantially different from that by which non-transmissible wild-type H5 virus HA binds human receptor. The structure of the complex also explains how the change in preference from avian to human receptors arises from the Gln226Leu substitution, which facilitates binding to human receptor but restricts binding to avian receptor. Both features probably contribute to the acquisition of transmissibility by this mutant virus.
- MRC National Institute for Medical Research, The Ridgeway, Mill Hill, London NW7 1AA, UK.
Organizational Affiliation: