Structural and Biochemical Characterization of the Broad Substrate Specificity of Bacteroides Thetaiotaomicron Commensal Sialidase.
Park, K., Kim, M., Ahn, H., Lee, D., Kim, J., Kim, Y., Woo, E.(2013) Biochim Biophys Acta 1834: 1510
- PubMed: 23665536
- DOI: https://doi.org/10.1016/j.bbapap.2013.04.028
- Primary Citation of Related Structures:
4BBW - PubMed Abstract:
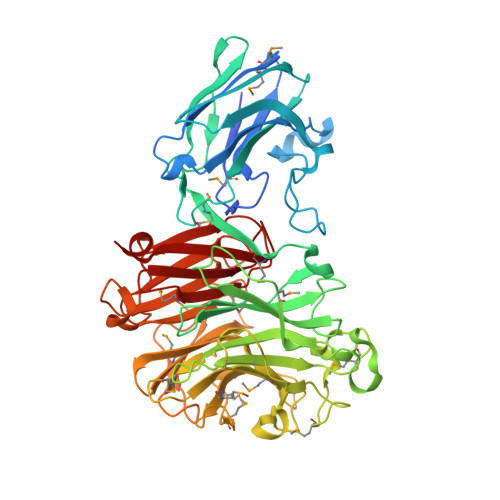
Sialidases release the terminal sialic acid residue from a wide range of sialic acid-containing polysaccharides. Bacteroides thetaiotaomicron, a symbiotic commensal microbe, resides in and dominates the human intestinal tract. We characterized the recombinant sialidase from B. thetaiotaomicron (BTSA) and demonstrated that it has broad substrate specificity with a relative activity of 97, 100 and 64 for 2,3-, 2,6- and 2,8-linked sialic substrates, respectively. The hydrolysis activity of BTSA was inhibited by a transition state analogue, 2-deoxy-2,3-dehydro-N-acetyl neuraminic acid, by competitive inhibition with a Ki value of 35μM. The structure of BSTA was determined at a resolution of 2.3Å. This structure exhibited a unique carbohydrate-binding domain (CBM) at its N-terminus (a.a. 23-190) that is adjacent to the catalytic domain (a.a. 191-535). The catalytic domain has a conserved arginine triad with a wide-open entrance for the substrate that exposes the catalytic residue to the surface. Unlike other pathogenic sialidases, the polysaccharide-binding site in the CBM is near the active site and possibly holds and positions the polysaccharide substrate directly at the active site. The structural feature of a wide substrate-binding groove and closer proximity of the polysaccharide-binding site to the active site could be a unique signature of the commensal sialidase BTSA and provide a molecular basis for its pharmaceutical application.
- Korea Research Institute of Bioscience & Biotechnology, Daejeon, Republic of Korea.
Organizational Affiliation: