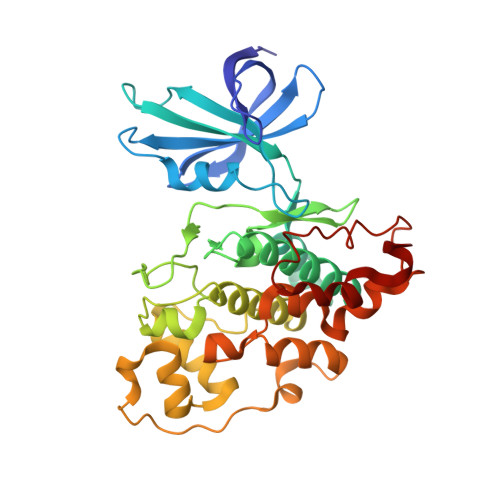
Selectivity, Cocrystal Structures, and Neuroprotective Properties of Leucettines, a Family of Protein Kinase Inhibitors Derived from the Marine Sponge Alkaloid Leucettamine B.
Tahtouh, T., Elkins, J.M., Filippakopoulos, P., Soundararajan, M., Burgy, G., Durieu, E., Cochet, C., Schmid, R.S., Lo, D.C., Delhommel, F., Oberholzer, A.E., Pearl, L.H., Carreaux, F., Bazureau, J., Knapp, S., Meijer, L.(2012) J Med Chem 55: 9312
- PubMed: 22998443
- DOI: https://doi.org/10.1021/jm301034u
- Primary Citation of Related Structures:
4AZE, 4AZF, 4B7T, 4GW8 - PubMed Abstract:
DYRKs (dual specificity, tyrosine phosphorylation regulated kinases) and CLKs (cdc2-like kinases) are implicated in the onset and development of Alzheimer's disease and Down syndrome. The marine sponge alkaloid leucettamine B was recently identified as an inhibitor of DYRKs/CLKs. Synthesis of analogues (leucettines) led to an optimized product, leucettine L41. Leucettines were cocrystallized with DYRK1A, DYRK2, CLK3, PIM1, and GSK-3β. The selectivity of L41 was studied by activity and interaction assays of recombinant kinases and affinity chromatography and competition affinity assays. These approaches revealed unexpected potential secondary targets such as CK2, SLK, and the lipid kinase PIKfyve/Vac14/Fig4. L41 displayed neuroprotective effects on glutamate-induced HT22 cell death. L41 also reduced amyloid precursor protein-induced cell death in cultured rat brain slices. The unusual multitarget selectivity of leucettines may account for their neuroprotective effects. This family of kinase inhibitors deserves further optimization as potential therapeutics against neurodegenerative diseases such as Alzheimer's disease.
- CNRS, "Protein Phosphorylation & Human Disease" Group, Station Biologique, 29680 Roscoff, Bretagne, France.
Organizational Affiliation: